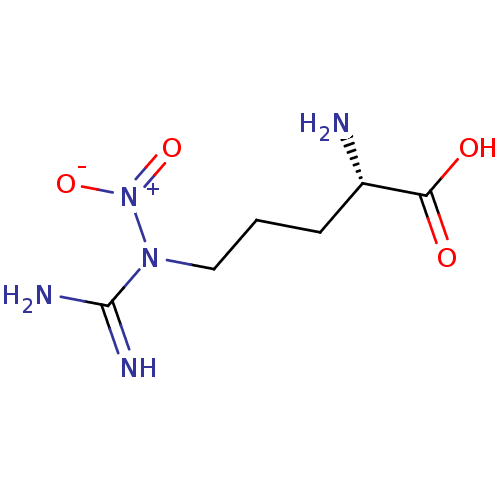
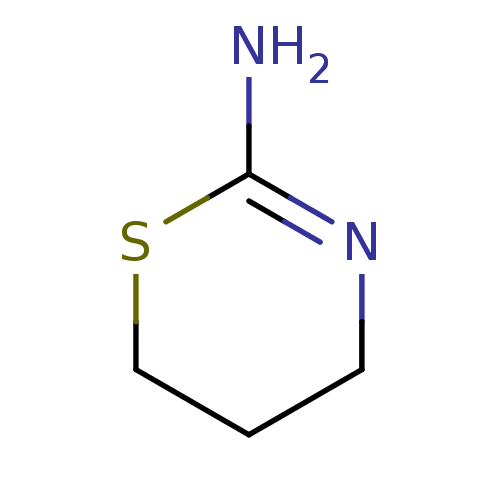
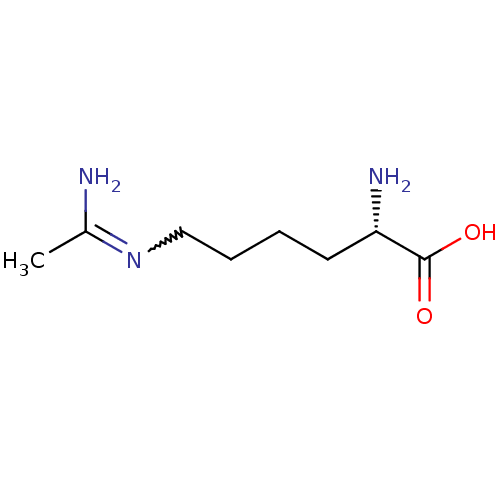
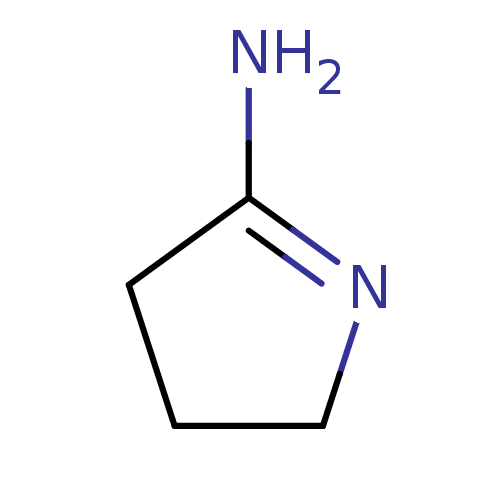
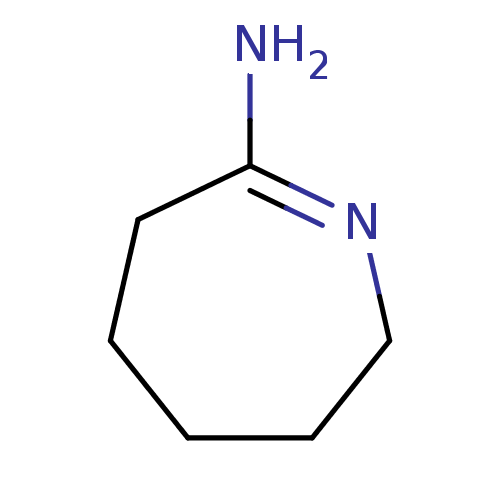TargetNitric oxide synthase, brain(Homo sapiens (Human))
G. D. Searle Research And Development
Curated by ChEMBL
G. D. Searle Research And Development
Curated by ChEMBL
Affinity DataIC50: 500nMAssay Description:Inhibitory activity against human Neuronal nitric oxide synthaseMore data for this Ligand-Target Pair
TargetNitric oxide synthase, endothelial(Homo sapiens (Human))
G. D. Searle Research And Development
Curated by ChEMBL
G. D. Searle Research And Development
Curated by ChEMBL
Affinity DataIC50: 500nMAssay Description:Inhibitory activity against human Endothelial nitric oxide synthaseMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Homo sapiens (Human))
G. D. Searle Research And Development
Curated by ChEMBL
G. D. Searle Research And Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibitory activity against human inducible nitric oxide synthaseMore data for this Ligand-Target Pair
TargetNitric oxide synthase, brain(Homo sapiens (Human))
G. D. Searle Research And Development
Curated by ChEMBL
G. D. Searle Research And Development
Curated by ChEMBL
Affinity DataIC50: 1.10E+3nMAssay Description:Inhibitory activity against human Neuronal nitric oxide synthaseMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Homo sapiens (Human))
G. D. Searle Research And Development
Curated by ChEMBL
G. D. Searle Research And Development
Curated by ChEMBL
Affinity DataIC50: 1.80E+3nMAssay Description:Inhibitory activity against human inducible nitric oxide synthaseMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Homo sapiens (Human))
G. D. Searle Research And Development
Curated by ChEMBL
G. D. Searle Research And Development
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibitory activity against human inducible nitric oxide synthaseMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Homo sapiens (Human))
G. D. Searle Research And Development
Curated by ChEMBL
G. D. Searle Research And Development
Curated by ChEMBL
Affinity DataIC50: 2.90E+3nMAssay Description:Inhibitory activity against human inducible nitric oxide synthaseMore data for this Ligand-Target Pair
TargetNitric oxide synthase, brain(Homo sapiens (Human))
G. D. Searle Research And Development
Curated by ChEMBL
G. D. Searle Research And Development
Curated by ChEMBL
Affinity DataIC50: 3.20E+3nMAssay Description:Inhibitory activity against human Neuronal nitric oxide synthaseMore data for this Ligand-Target Pair
TargetNitric oxide synthase, brain(Homo sapiens (Human))
G. D. Searle Research And Development
Curated by ChEMBL
G. D. Searle Research And Development
Curated by ChEMBL
Affinity DataIC50: 3.50E+3nMAssay Description:Inhibitory activity against human Neuronal nitric oxide synthaseMore data for this Ligand-Target Pair
TargetNitric oxide synthase, brain(Homo sapiens (Human))
G. D. Searle Research And Development
Curated by ChEMBL
G. D. Searle Research And Development
Curated by ChEMBL
Affinity DataIC50: 3.70E+3nMAssay Description:Inhibitory activity against human Neuronal nitric oxide synthaseMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Homo sapiens (Human))
G. D. Searle Research And Development
Curated by ChEMBL
G. D. Searle Research And Development
Curated by ChEMBL
Affinity DataIC50: 4.60E+3nMAssay Description:Inhibitory activity against human inducible nitric oxide synthaseMore data for this Ligand-Target Pair
TargetNitric oxide synthase, endothelial(Homo sapiens (Human))
G. D. Searle Research And Development
Curated by ChEMBL
G. D. Searle Research And Development
Curated by ChEMBL
Affinity DataIC50: 4.70E+3nMAssay Description:Inhibitory activity against human Endothelial nitric oxide synthaseMore data for this Ligand-Target Pair
TargetNitric oxide synthase, endothelial(Homo sapiens (Human))
G. D. Searle Research And Development
Curated by ChEMBL
G. D. Searle Research And Development
Curated by ChEMBL
Affinity DataIC50: 5.90E+3nMAssay Description:Inhibitory activity against human Endothelial nitric oxide synthaseMore data for this Ligand-Target Pair
TargetNitric oxide synthase, brain(Homo sapiens (Human))
G. D. Searle Research And Development
Curated by ChEMBL
G. D. Searle Research And Development
Curated by ChEMBL
Affinity DataIC50: 6.80E+3nMAssay Description:Inhibitory activity against human Neuronal nitric oxide synthaseMore data for this Ligand-Target Pair
TargetNitric oxide synthase, endothelial(Homo sapiens (Human))
G. D. Searle Research And Development
Curated by ChEMBL
G. D. Searle Research And Development
Curated by ChEMBL
Affinity DataIC50: 7.10E+3nMAssay Description:Inhibitory activity against human Endothelial nitric oxide synthaseMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Homo sapiens (Human))
G. D. Searle Research And Development
Curated by ChEMBL
G. D. Searle Research And Development
Curated by ChEMBL
Affinity DataIC50: 7.60E+3nMAssay Description:Inhibitory activity against human inducible nitric oxide synthaseMore data for this Ligand-Target Pair
TargetNitric oxide synthase, endothelial(Homo sapiens (Human))
G. D. Searle Research And Development
Curated by ChEMBL
G. D. Searle Research And Development
Curated by ChEMBL
Affinity DataIC50: 8.20E+3nMAssay Description:Inhibitory activity against human Endothelial nitric oxide synthaseMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Homo sapiens (Human))
G. D. Searle Research And Development
Curated by ChEMBL
G. D. Searle Research And Development
Curated by ChEMBL
Affinity DataIC50: 9.80E+3nMAssay Description:Inhibitory activity against human inducible nitric oxide synthaseMore data for this Ligand-Target Pair
TargetNitric oxide synthase, brain(Homo sapiens (Human))
G. D. Searle Research And Development
Curated by ChEMBL
G. D. Searle Research And Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibitory activity against human Neuronal nitric oxide synthaseMore data for this Ligand-Target Pair
TargetNitric oxide synthase, brain(Homo sapiens (Human))
G. D. Searle Research And Development
Curated by ChEMBL
G. D. Searle Research And Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibitory activity against human Neuronal nitric oxide synthaseMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Homo sapiens (Human))
G. D. Searle Research And Development
Curated by ChEMBL
G. D. Searle Research And Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibitory activity against human inducible nitric oxide synthaseMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Homo sapiens (Human))
G. D. Searle Research And Development
Curated by ChEMBL
G. D. Searle Research And Development
Curated by ChEMBL
Affinity DataIC50: 1.40E+4nMAssay Description:Inhibitory activity against human inducible nitric oxide synthaseMore data for this Ligand-Target Pair
TargetNitric oxide synthase, endothelial(Homo sapiens (Human))
G. D. Searle Research And Development
Curated by ChEMBL
G. D. Searle Research And Development
Curated by ChEMBL
Affinity DataIC50: 1.60E+4nMAssay Description:Inhibitory activity against human Endothelial nitric oxide synthaseMore data for this Ligand-Target Pair
TargetNitric oxide synthase, brain(Homo sapiens (Human))
G. D. Searle Research And Development
Curated by ChEMBL
G. D. Searle Research And Development
Curated by ChEMBL
Affinity DataIC50: 1.70E+4nMAssay Description:Inhibitory activity against human Neuronal nitric oxide synthaseMore data for this Ligand-Target Pair
TargetNitric oxide synthase, brain(Homo sapiens (Human))
G. D. Searle Research And Development
Curated by ChEMBL
G. D. Searle Research And Development
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibitory activity against human Neuronal nitric oxide synthaseMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Homo sapiens (Human))
G. D. Searle Research And Development
Curated by ChEMBL
G. D. Searle Research And Development
Curated by ChEMBL
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibitory activity against human inducible nitric oxide synthaseMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Homo sapiens (Human))
G. D. Searle Research And Development
Curated by ChEMBL
G. D. Searle Research And Development
Curated by ChEMBL
Affinity DataIC50: 2.60E+4nMAssay Description:Inhibitory activity against human inducible nitric oxide synthaseMore data for this Ligand-Target Pair
TargetNitric oxide synthase, endothelial(Homo sapiens (Human))
G. D. Searle Research And Development
Curated by ChEMBL
G. D. Searle Research And Development
Curated by ChEMBL
Affinity DataIC50: 5.90E+4nMAssay Description:Inhibitory activity against human Endothelial nitric oxide synthaseMore data for this Ligand-Target Pair
TargetNitric oxide synthase, brain(Homo sapiens (Human))
G. D. Searle Research And Development
Curated by ChEMBL
G. D. Searle Research And Development
Curated by ChEMBL
Affinity DataIC50: 6.10E+4nMAssay Description:Inhibitory activity against human Neuronal nitric oxide synthaseMore data for this Ligand-Target Pair
TargetNitric oxide synthase, endothelial(Homo sapiens (Human))
G. D. Searle Research And Development
Curated by ChEMBL
G. D. Searle Research And Development
Curated by ChEMBL
Affinity DataIC50: 8.70E+4nMAssay Description:Inhibitory activity against human Endothelial nitric oxide synthaseMore data for this Ligand-Target Pair
TargetNitric oxide synthase, endothelial(Homo sapiens (Human))
G. D. Searle Research And Development
Curated by ChEMBL
G. D. Searle Research And Development
Curated by ChEMBL
Affinity DataIC50: 9.00E+4nMAssay Description:Inhibitory activity against human Endothelial nitric oxide synthaseMore data for this Ligand-Target Pair
TargetNitric oxide synthase, endothelial(Homo sapiens (Human))
G. D. Searle Research And Development
Curated by ChEMBL
G. D. Searle Research And Development
Curated by ChEMBL
Affinity DataIC50: 9.30E+4nMAssay Description:Inhibitory activity against human Endothelial nitric oxide synthaseMore data for this Ligand-Target Pair
TargetNitric oxide synthase, endothelial(Homo sapiens (Human))
G. D. Searle Research And Development
Curated by ChEMBL
G. D. Searle Research And Development
Curated by ChEMBL
Affinity DataIC50: 1.38E+5nMAssay Description:Inhibitory activity against human Endothelial nitric oxide synthaseMore data for this Ligand-Target Pair