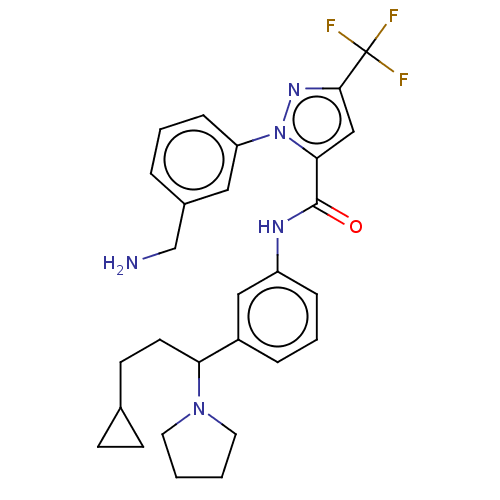
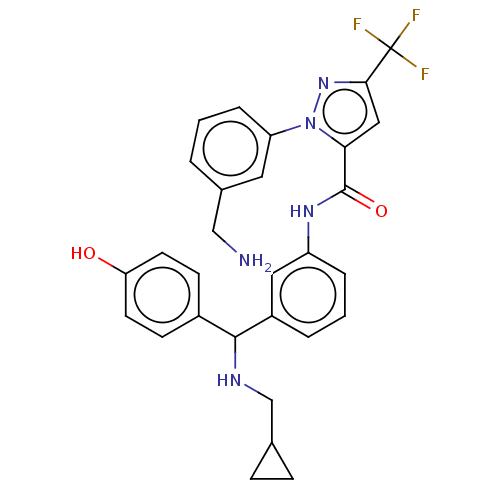
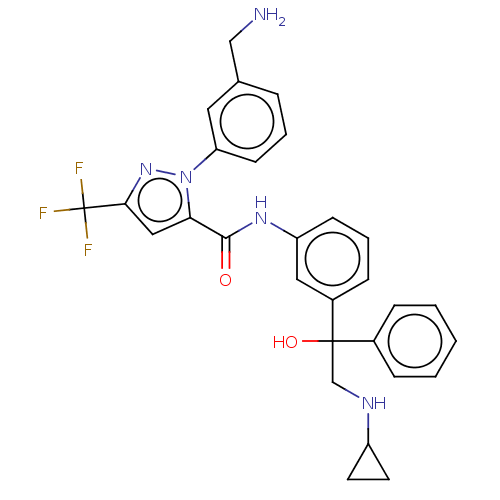
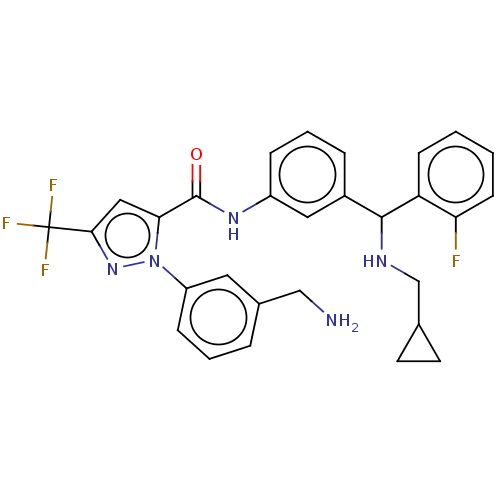
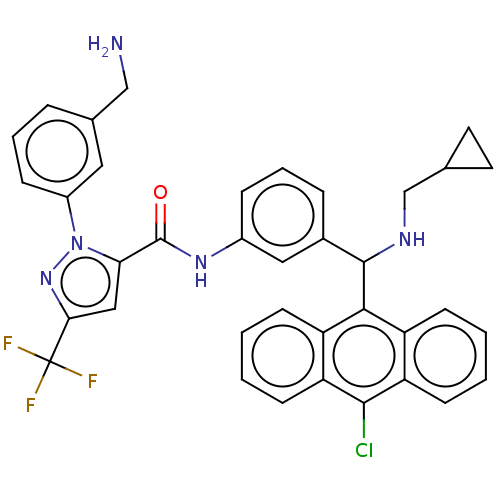
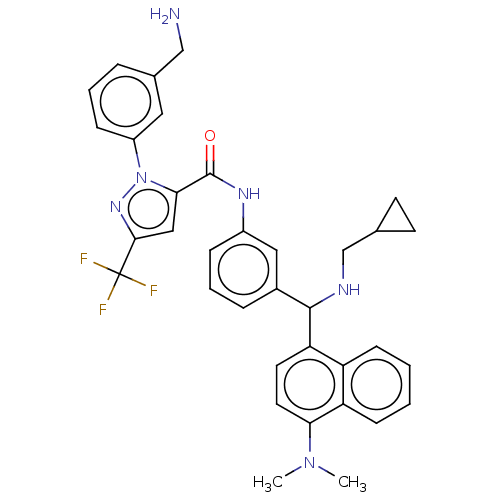
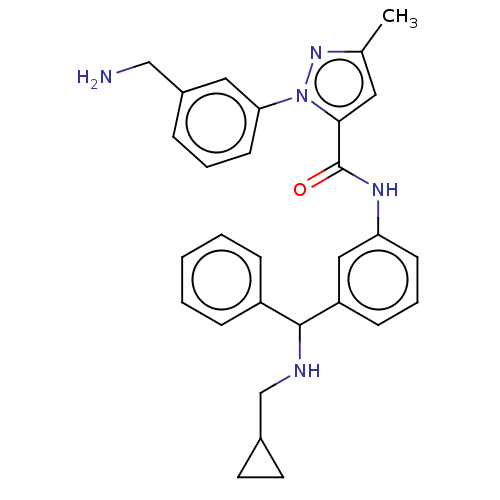
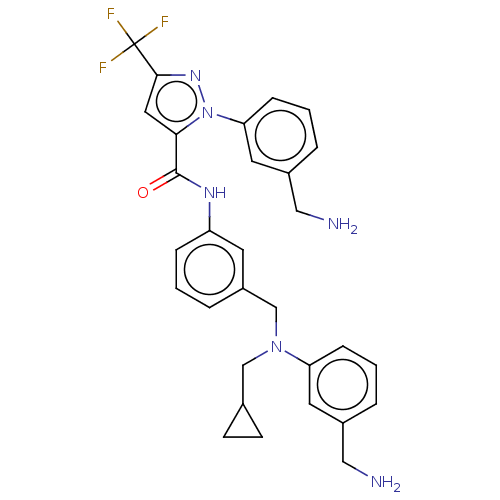
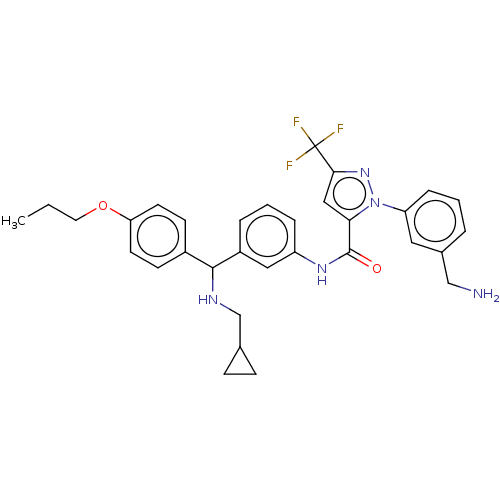
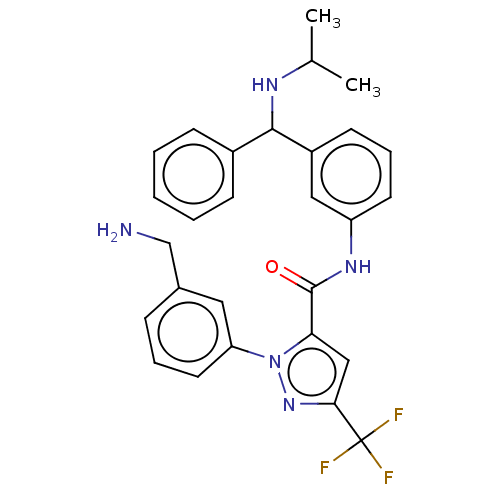
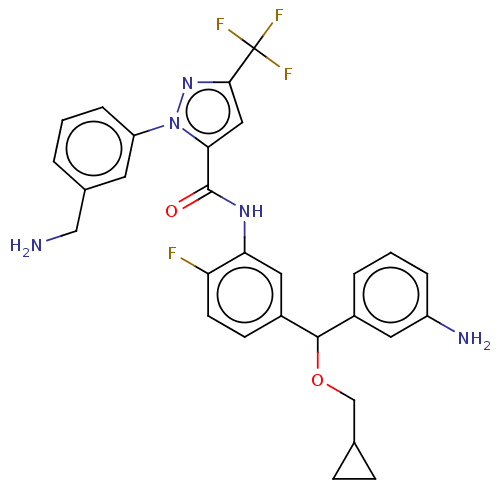
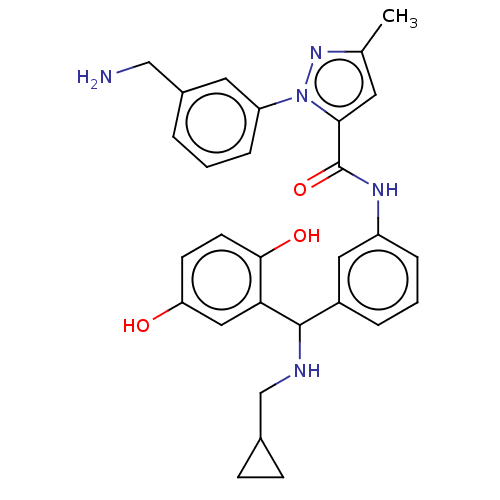
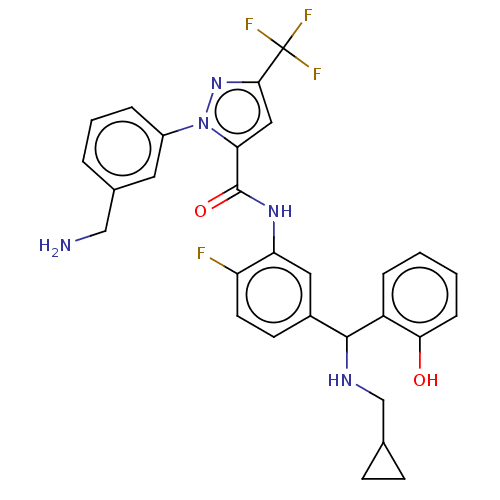
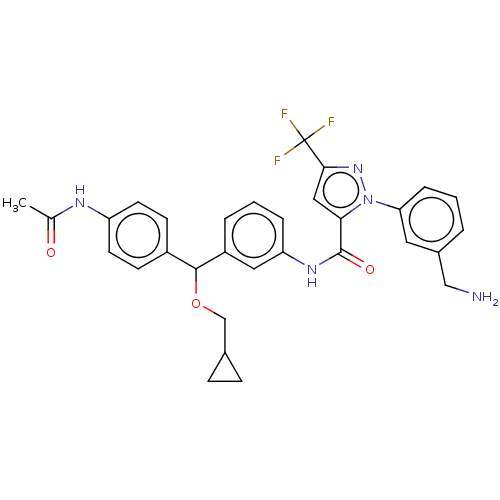
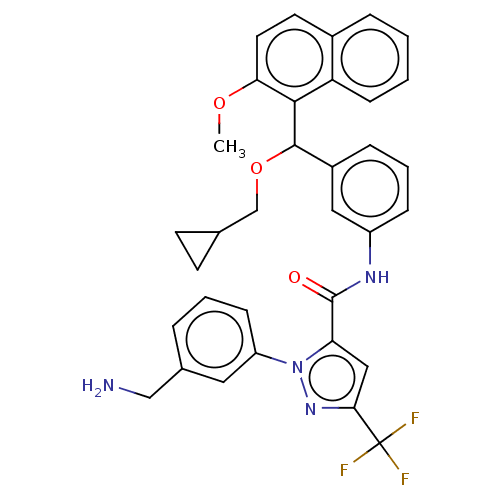
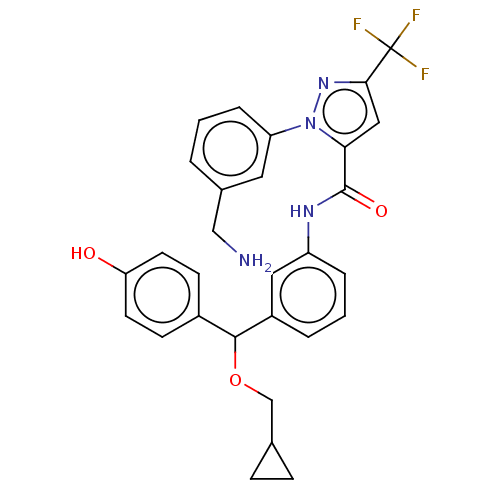
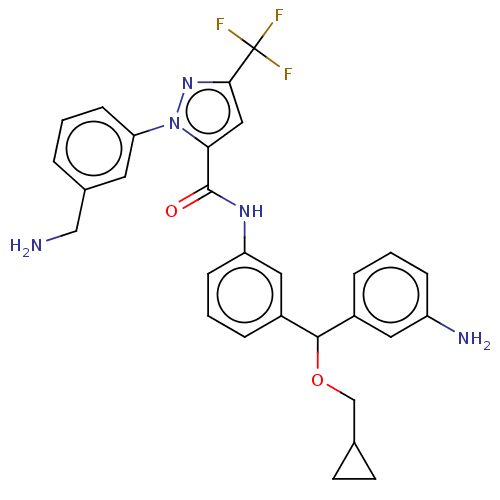
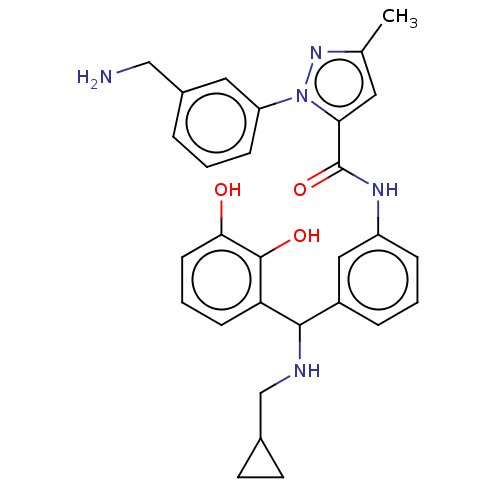
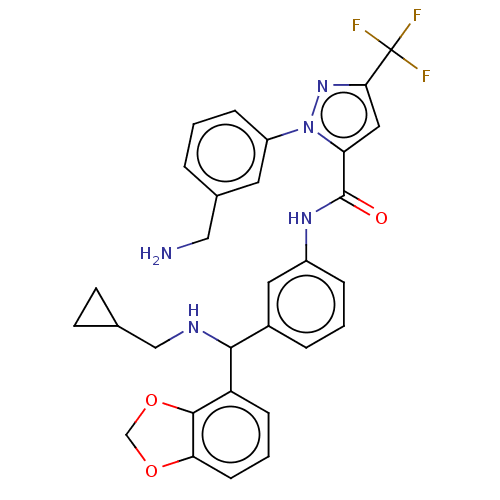
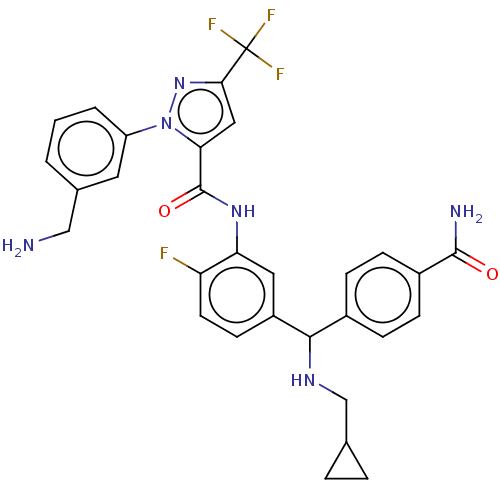
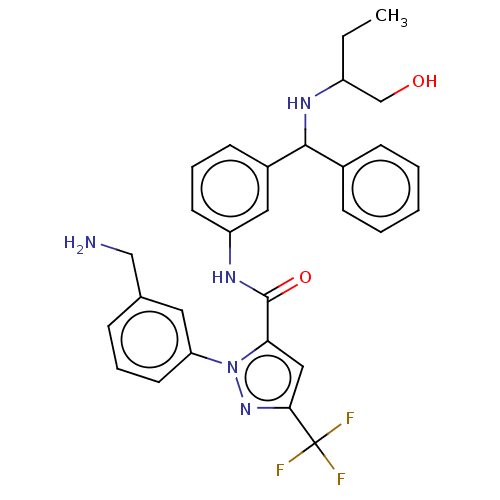
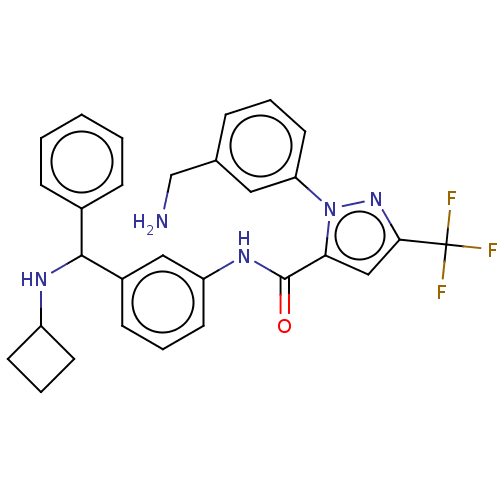
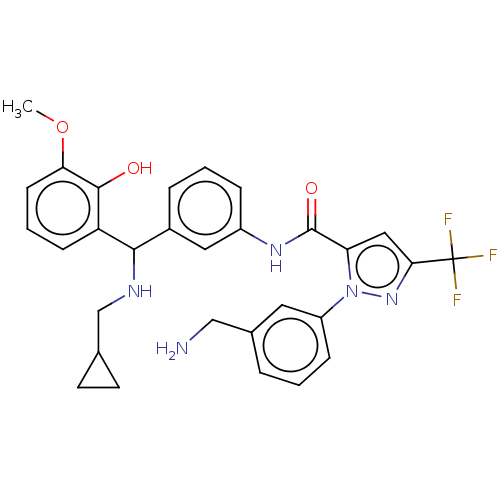
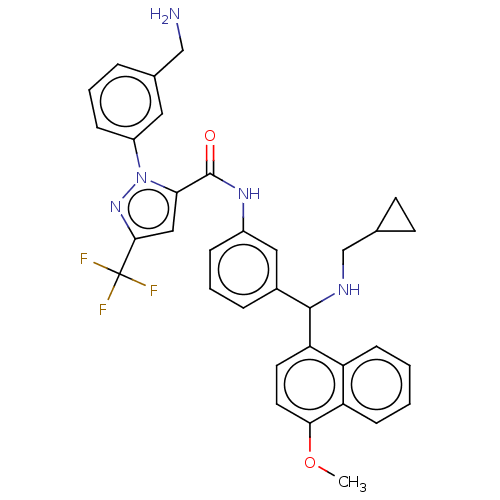
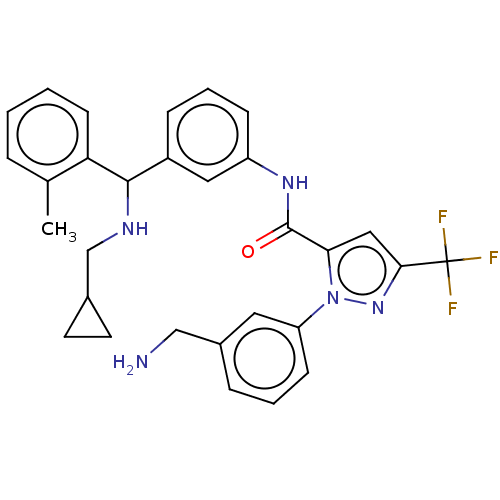
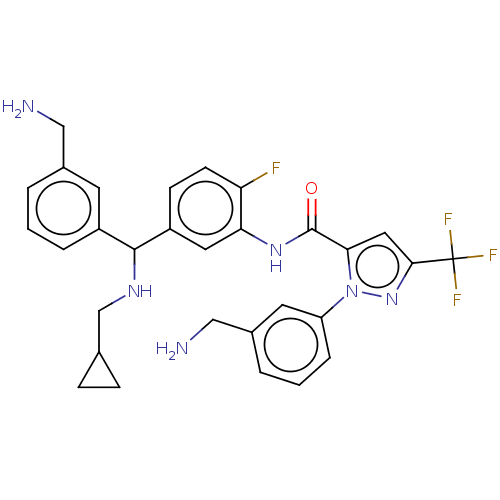
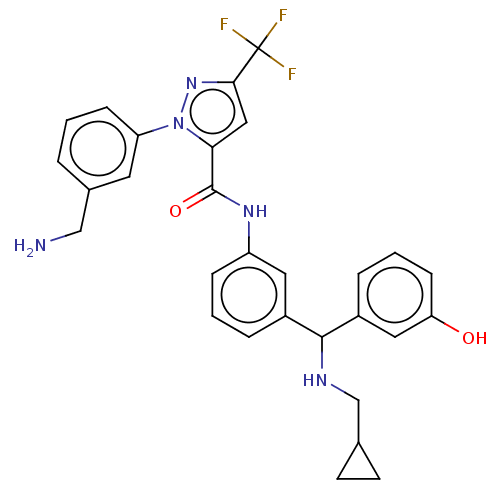
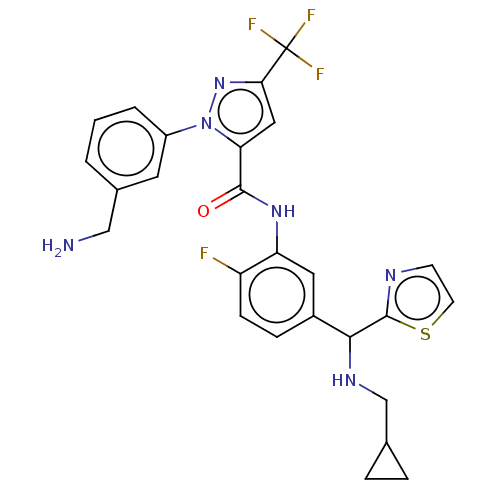
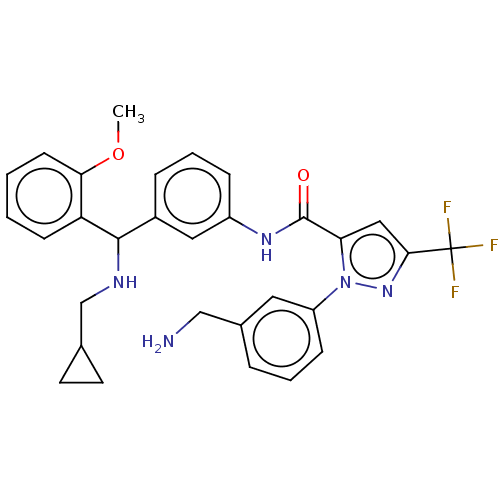
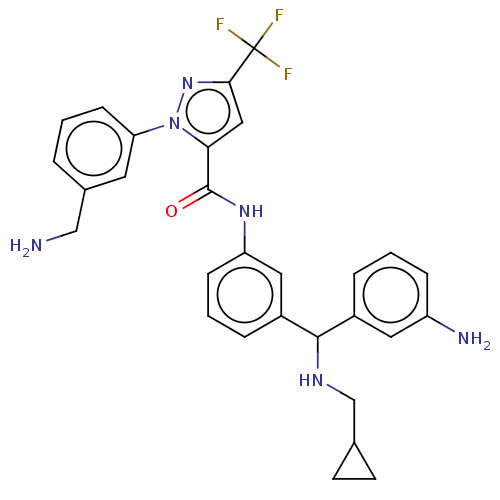
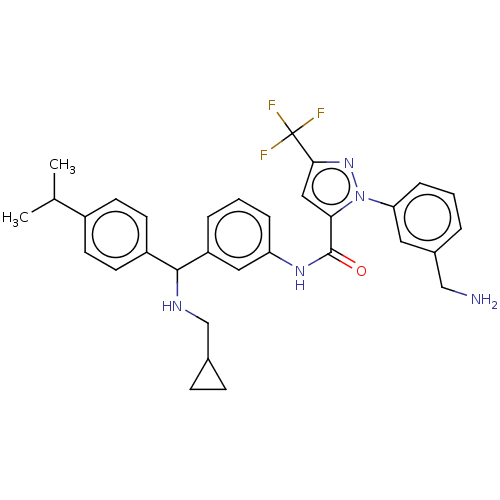
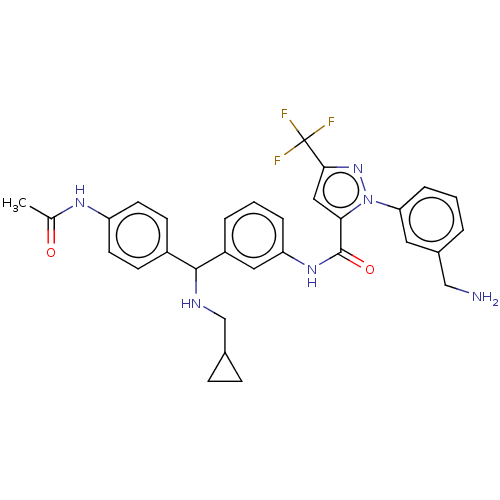
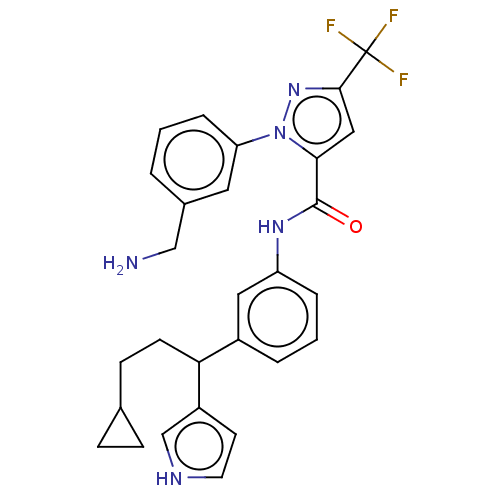
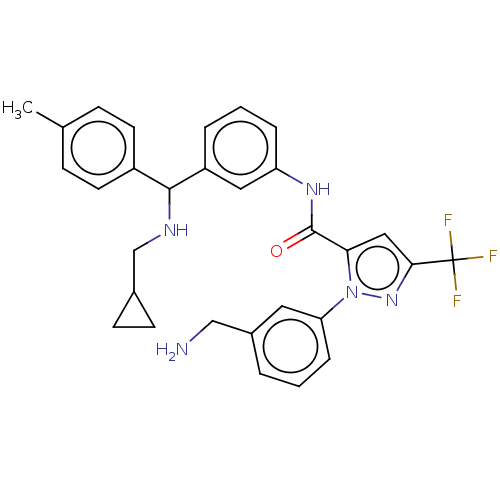
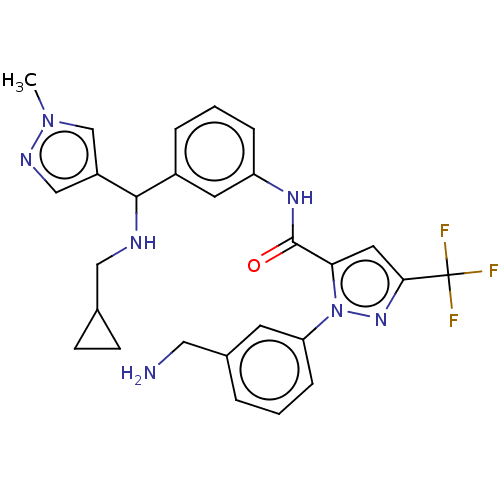
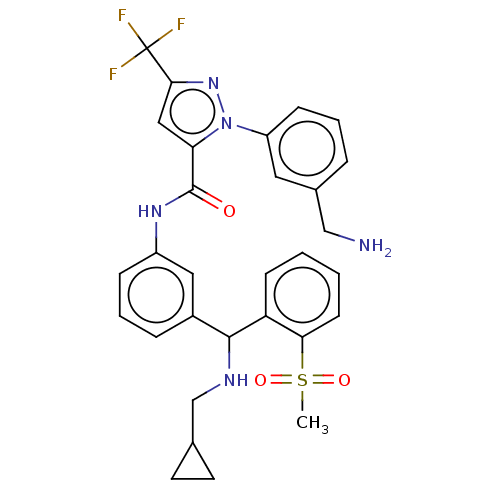
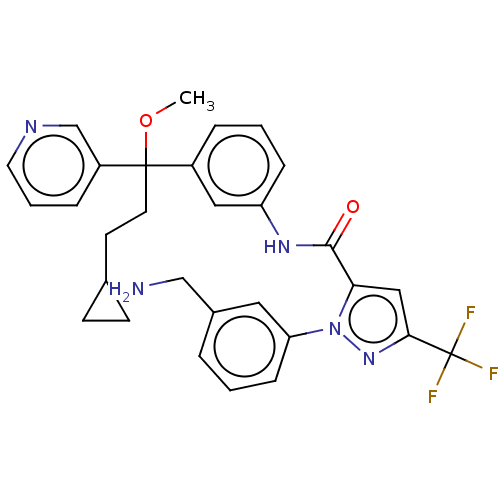
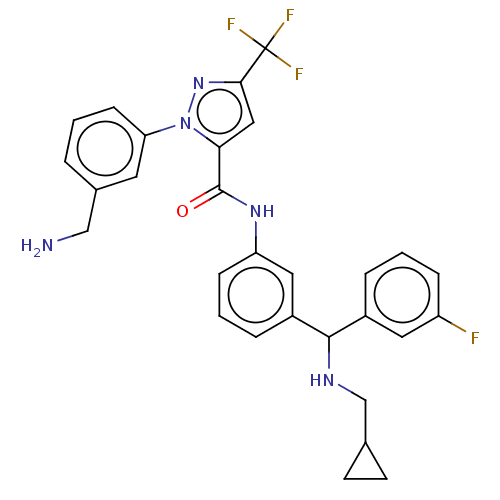
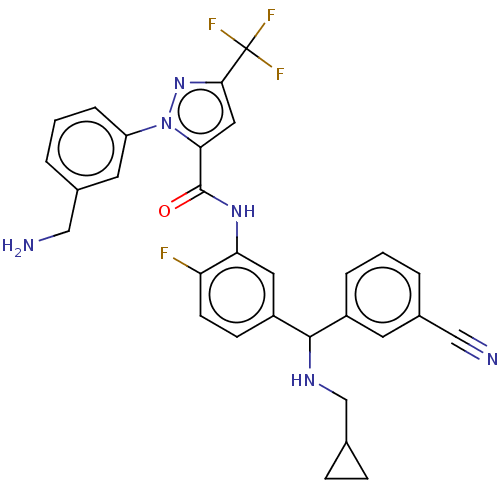
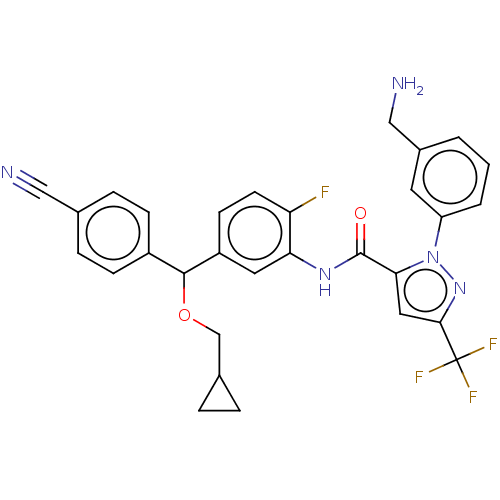
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair