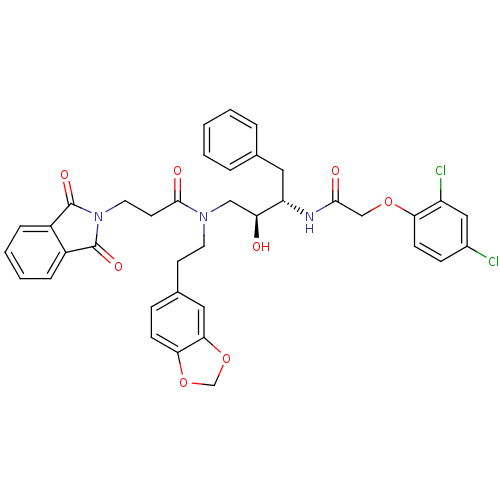
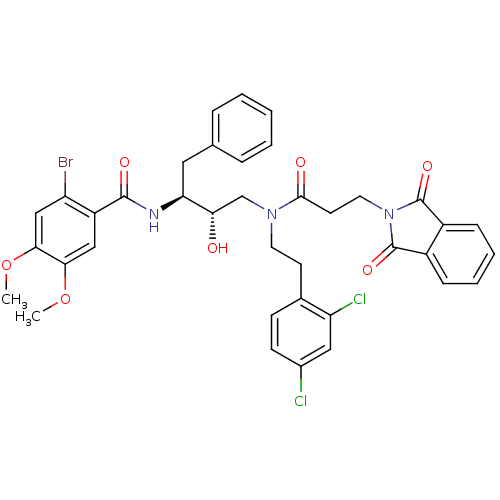
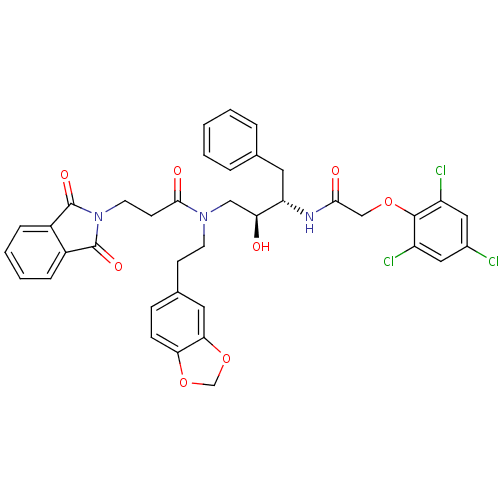
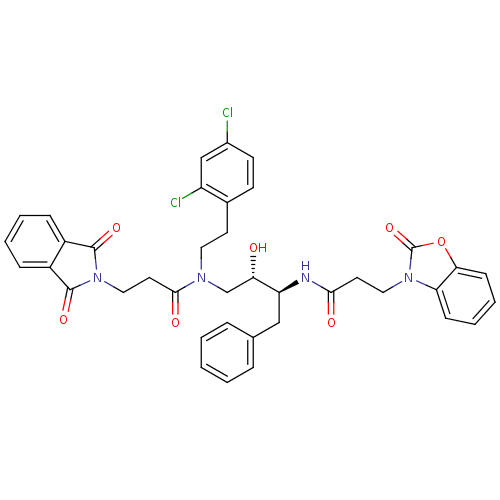
Affinity DataKi: 3nMAssay Description:Inhibitory activity of compound against human Cathepsin DMore data for this Ligand-Target Pair
Affinity DataKi: 5.20nMAssay Description:Inhibitory activity of compound against human Cathepsin DMore data for this Ligand-Target Pair
Affinity DataKi: 73nMAssay Description:Inhibitory activity of compound against human Cathepsin DMore data for this Ligand-Target Pair
Affinity DataKi: 231nMAssay Description:Inhibitory activity of compound against human Cathepsin DMore data for this Ligand-Target Pair
Affinity DataKi: >5.00E+3nMAssay Description:Inhibitory activity of compound against human Cathepsin DMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Inhibitory activity of compound against human Cathepsin DMore data for this Ligand-Target Pair