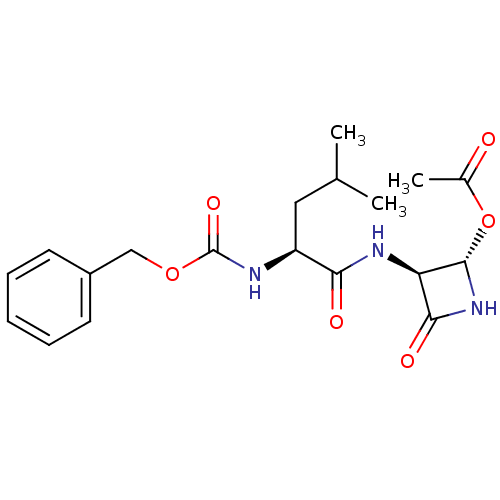
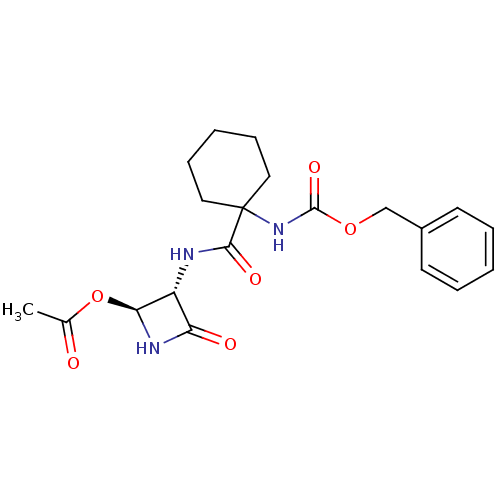
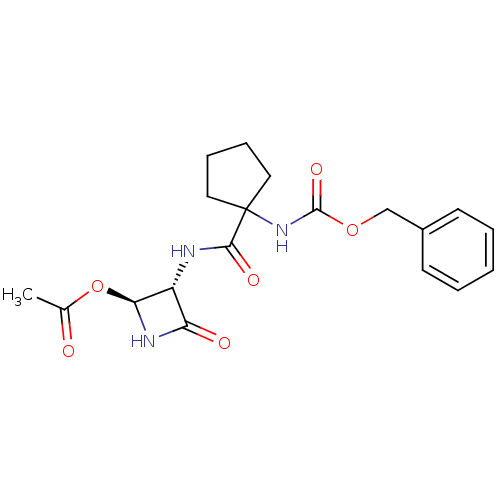
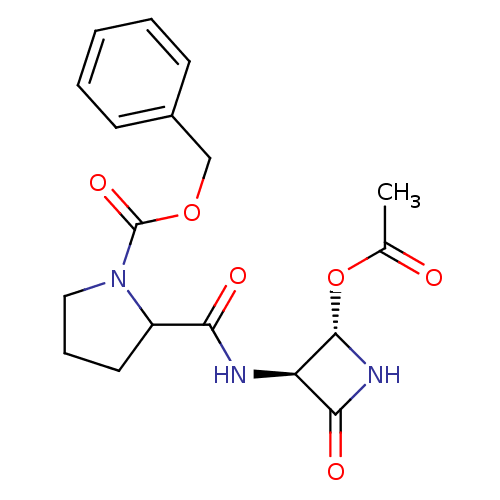
Affinity DataKi: 2.20nMAssay Description:Inhibitory activity of the compound was measured against Cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 5.70nMAssay Description:Inhibitory activity of the compound was measured against Cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 8.80nMAssay Description:Inhibitory activity of the compound was measured against Cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 9.20nMAssay Description:Inhibitory activity of the compound was measured against Cathepsin SMore data for this Ligand-Target Pair
Affinity DataKi: 88nMAssay Description:Inhibitory activity of the compound was measured against Cathepsin LMore data for this Ligand-Target Pair
Affinity DataKi: 130nMAssay Description:Inhibitory activity of the compound was measured against Cathepsin SMore data for this Ligand-Target Pair
Affinity DataKi: 160nMAssay Description:Inhibitory activity of the compound was measured against Cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 200nMAssay Description:Inhibitory activity of the compound was measured against Cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 380nMAssay Description:Inhibitory activity of the compound was measured against Cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 950nMAssay Description:Inhibitory activity of the compound was measured against Cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 950nMAssay Description:Inhibitory activity of the compound was measured against Cathepsin SMore data for this Ligand-Target Pair
Affinity DataKi: 1.60E+3nMAssay Description:Inhibitory activity of the compound was measured against Cathepsin BMore data for this Ligand-Target Pair
Affinity DataKi: 2.10E+3nMAssay Description:Inhibitory activity of the compound was measured against Cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 2.10E+3nMAssay Description:Inhibitory activity of the compound was measured against Cathepsin BMore data for this Ligand-Target Pair
Affinity DataKi: 3.40E+3nMAssay Description:Inhibitory activity of the compound was measured against Cathepsin LMore data for this Ligand-Target Pair
Affinity DataKi: 3.60E+3nMAssay Description:Inhibitory activity of the compound was measured against Cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 3.70E+3nMAssay Description:Inhibitory activity of the compound was measured against Cathepsin LMore data for this Ligand-Target Pair
Affinity DataKi: 4.30E+3nMAssay Description:Inhibitory activity of the compound was measured against Cathepsin BMore data for this Ligand-Target Pair
Affinity DataKi: 6.80E+3nMAssay Description:Inhibitory activity of the compound was measured against Cathepsin LMore data for this Ligand-Target Pair
Affinity DataKi: 7.70E+3nMAssay Description:Inhibitory activity of the compound was measured against Cathepsin BMore data for this Ligand-Target Pair
Affinity DataKi: 1.10E+4nMAssay Description:Inhibitory activity of the compound was measured against Cathepsin SMore data for this Ligand-Target Pair
Affinity DataKi: 1.60E+4nMAssay Description:Inhibitory activity of the compound was measured against Cathepsin LMore data for this Ligand-Target Pair
Affinity DataKi: 2.00E+4nMAssay Description:Inhibitory activity of the compound was measured against Cathepsin SMore data for this Ligand-Target Pair
Affinity DataKi: 2.80E+4nMAssay Description:Inhibitory activity of the compound was measured against Cathepsin SMore data for this Ligand-Target Pair
Affinity DataKi: 3.30E+4nMAssay Description:Inhibitory activity of the compound was measured against Cathepsin SMore data for this Ligand-Target Pair
Affinity DataKi: 5.20E+4nMAssay Description:Inhibitory activity of the compound was measured against Cathepsin BMore data for this Ligand-Target Pair
Affinity DataKi: 5.30E+4nMAssay Description:Inhibitory activity of the compound was measured against Cathepsin SMore data for this Ligand-Target Pair
Affinity DataKi: 6.50E+4nMAssay Description:Inhibitory activity of the compound was measured against Cathepsin BMore data for this Ligand-Target Pair
Affinity DataKi: 1.20E+5nMAssay Description:Inhibitory activity of the compound was measured against Cathepsin BMore data for this Ligand-Target Pair
Affinity DataKi: 1.40E+5nMAssay Description:Inhibitory activity of the compound was measured against Cathepsin BMore data for this Ligand-Target Pair
Affinity DataKi: 1.40E+5nMAssay Description:Inhibitory activity of the compound was measured against Cathepsin LMore data for this Ligand-Target Pair
Affinity DataKi: >1.50E+5nMAssay Description:Inhibitory activity of the compound was measured against Cathepsin LMore data for this Ligand-Target Pair
Affinity DataKi: >1.50E+5nMAssay Description:Inhibitory activity of the compound was measured against Cathepsin LMore data for this Ligand-Target Pair
Affinity DataKi: >1.50E+5nMAssay Description:Inhibitory activity of the compound was measured against Cathepsin BMore data for this Ligand-Target Pair
Affinity DataKi: >1.50E+5nMAssay Description:Inhibitory activity of the compound was measured against Cathepsin LMore data for this Ligand-Target Pair
Affinity DataKi: >1.50E+5nMAssay Description:Inhibitory activity of the compound was measured against Cathepsin SMore data for this Ligand-Target Pair