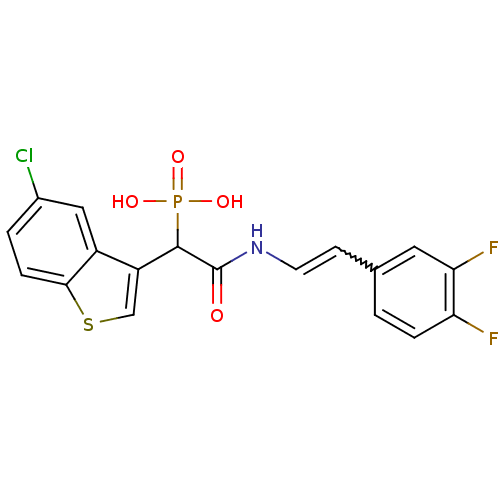
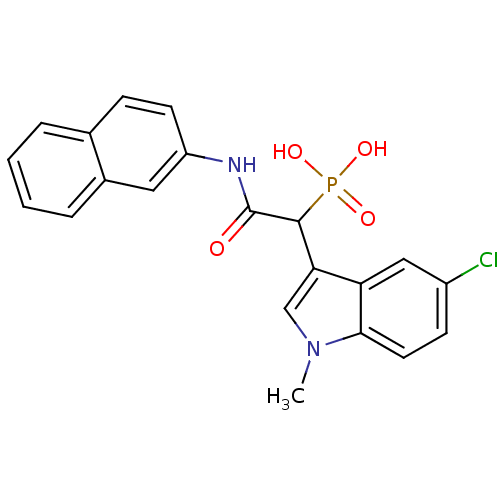
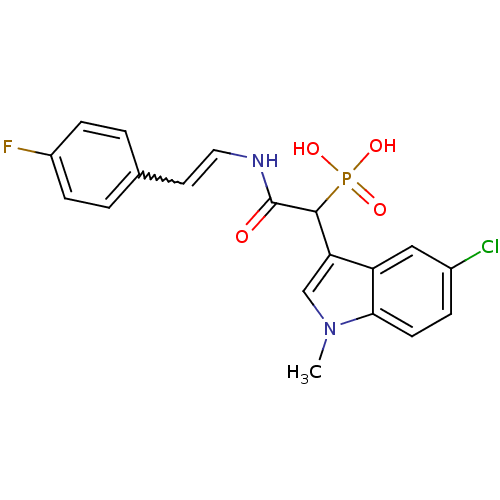
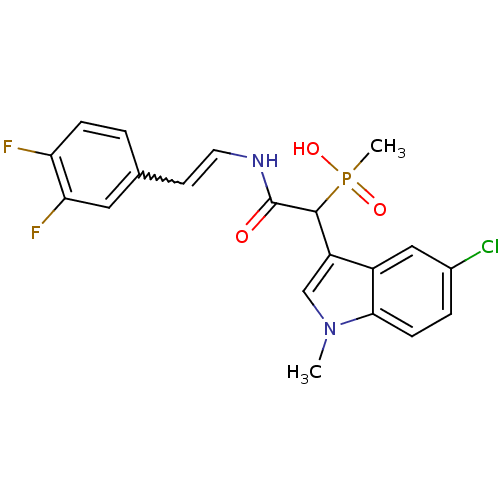
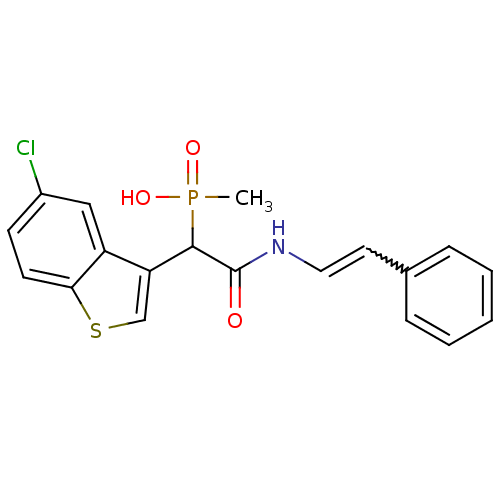
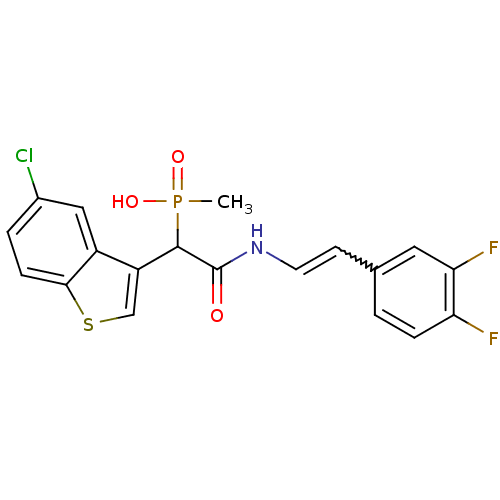
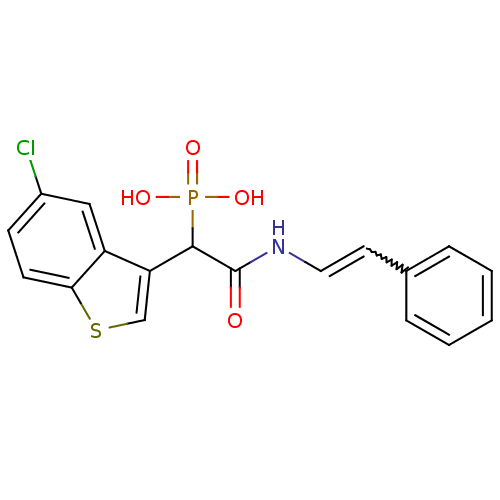
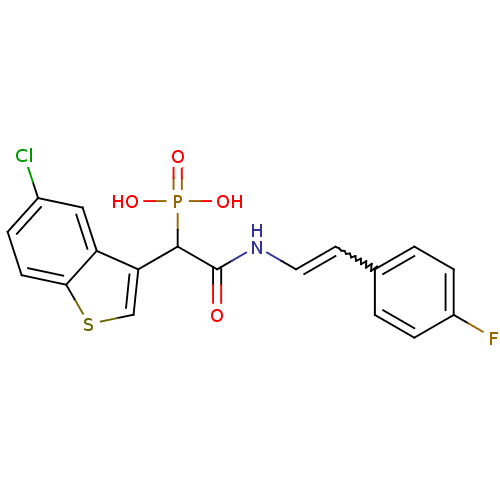
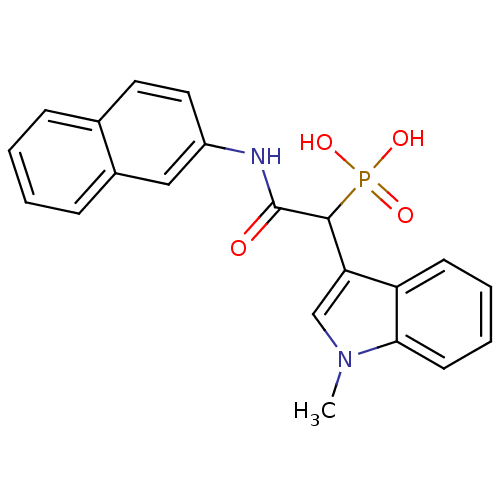
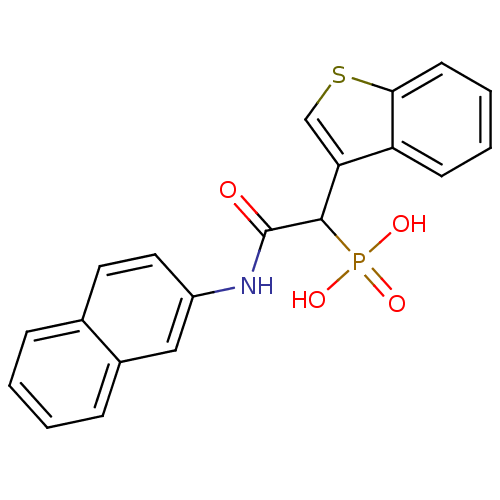
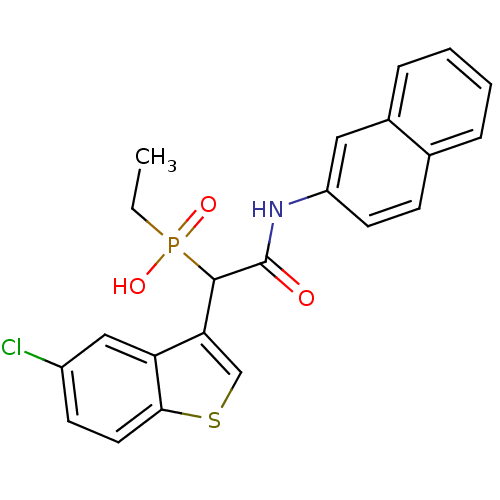
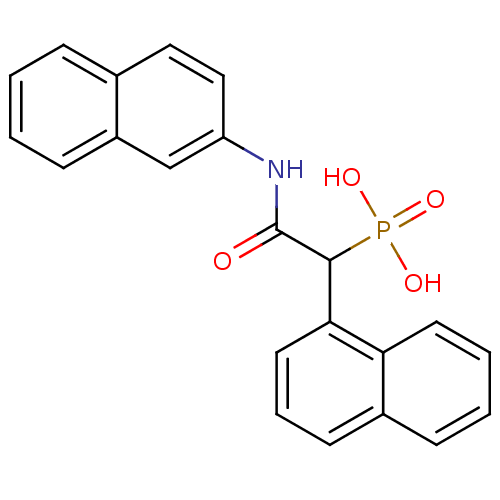
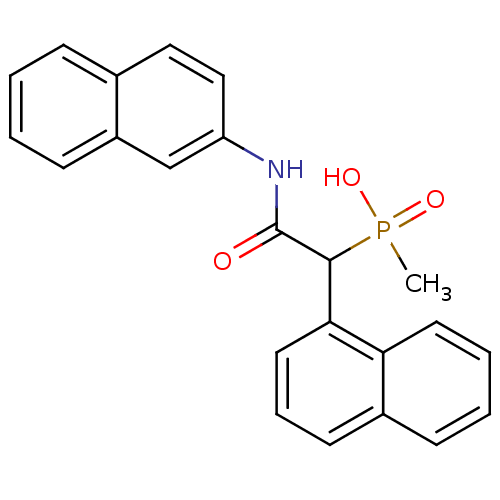
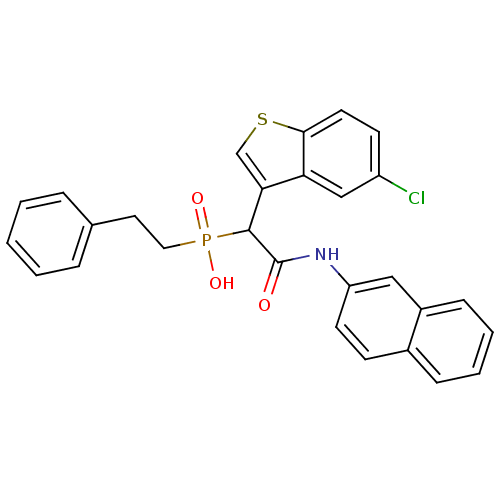
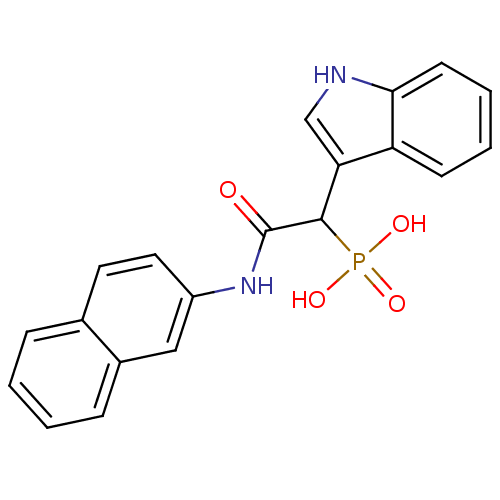
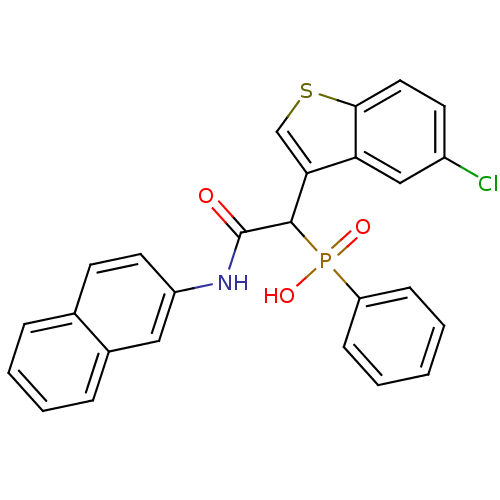
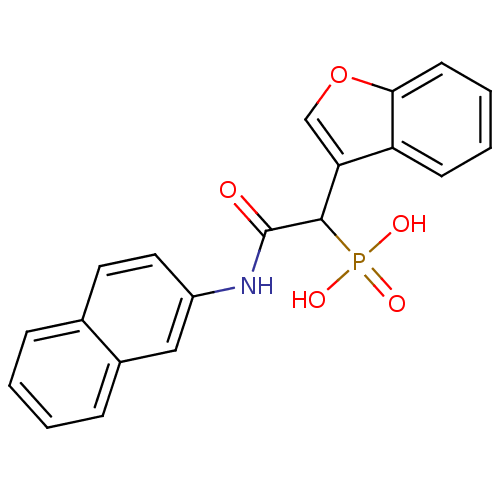
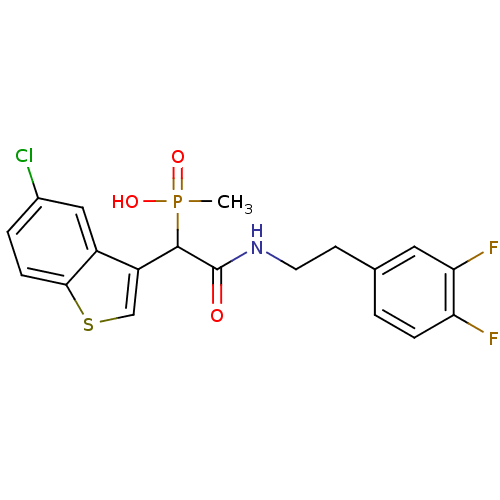
Report error Found 31 Enz. Inhib. hit(s) with all data for entry = 50019839

Affinity DataKi: 2.30nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
Affinity DataIC50: 3.5nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
Affinity DataIC50: 4.5nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
Affinity DataIC50: 11nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
Affinity DataKi: 11nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
Affinity DataIC50: 17nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
Affinity DataIC50: 21nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
Affinity DataIC50: 29nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
Affinity DataKi: 36nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
Affinity DataKi: 38nMAssay Description:Inhibition of human neutrophil Cat GMore data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
Affinity DataIC50: 58nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
Affinity DataIC50: 60nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
Affinity DataIC50: 66nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
Affinity DataIC50: 80nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
Affinity DataIC50: 90nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
Affinity DataIC50: 120nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
Affinity DataIC50: 160nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
Affinity DataIC50: 165nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
Affinity DataIC50: 190nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
Affinity DataIC50: 210nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
Affinity DataIC50: 240nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
Affinity DataIC50: 280nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
Affinity DataIC50: 920nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
Affinity DataIC50: 2.10E+3nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+3nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
Affinity DataKi: 9.50E+3nMAssay Description:Inhibition of human neutrophil Cat GMore data for this Ligand-Target Pair
Affinity DataIC50: 1.04E+4nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
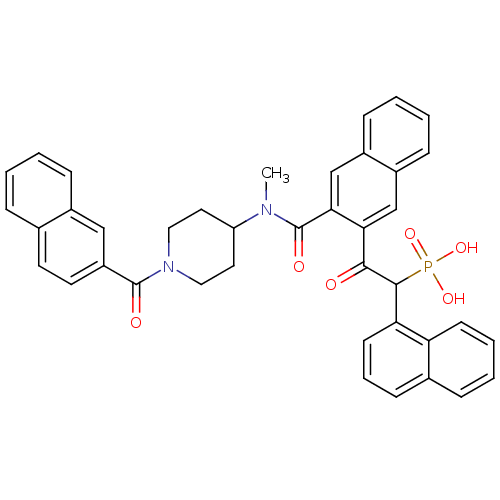
 3D Structure (crystal)
3D Structure (crystal)