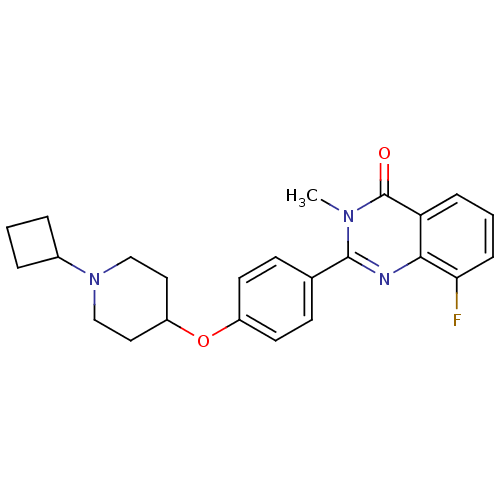
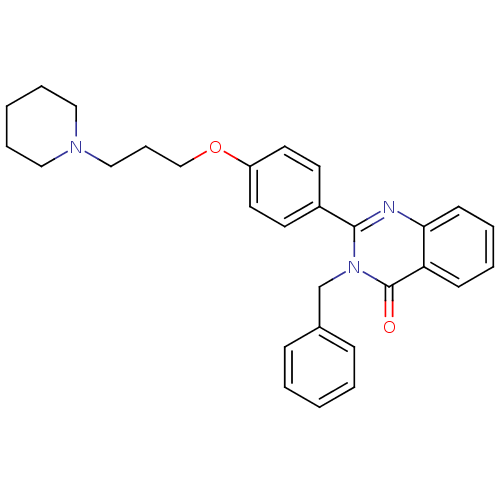
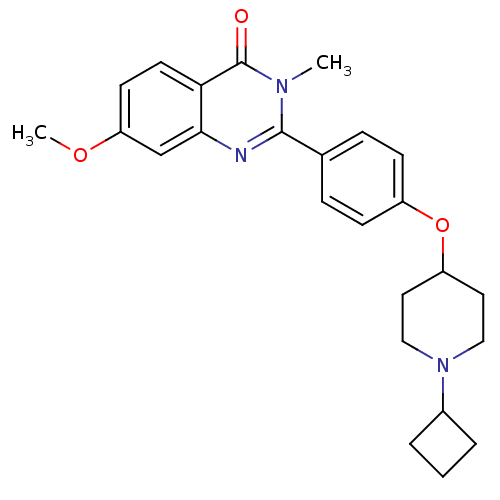
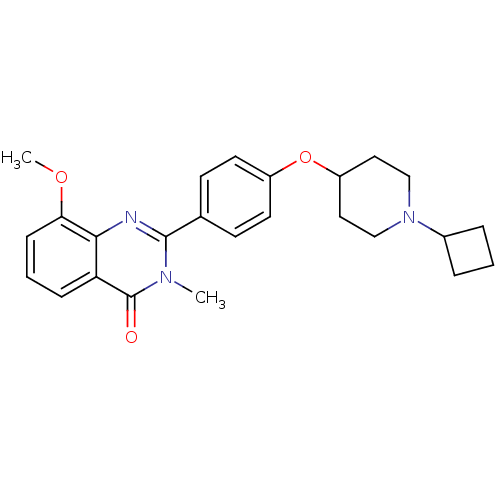
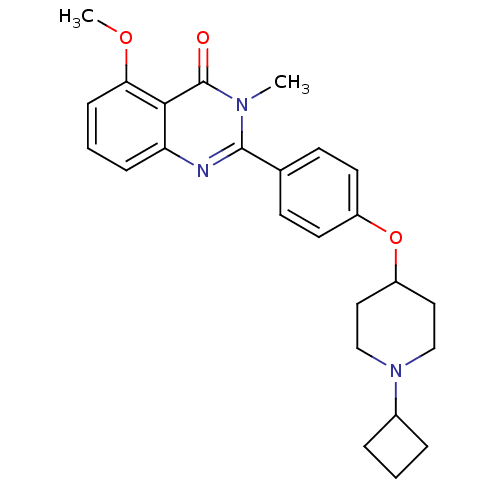
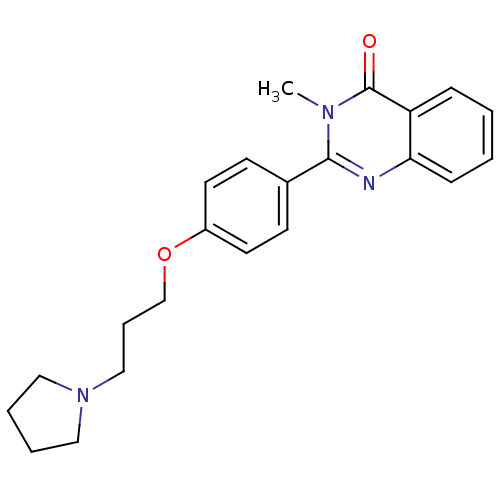
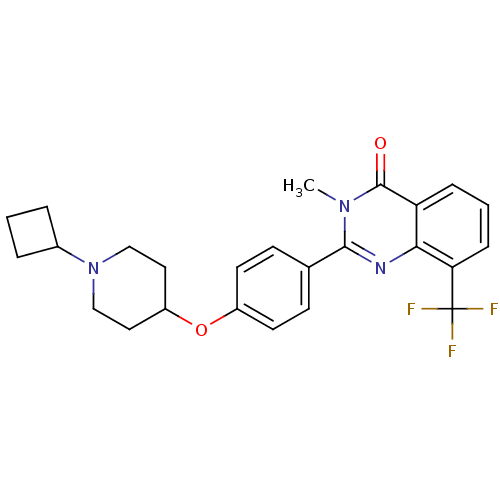
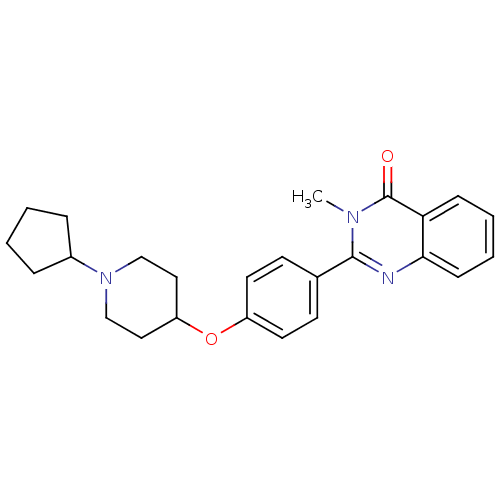
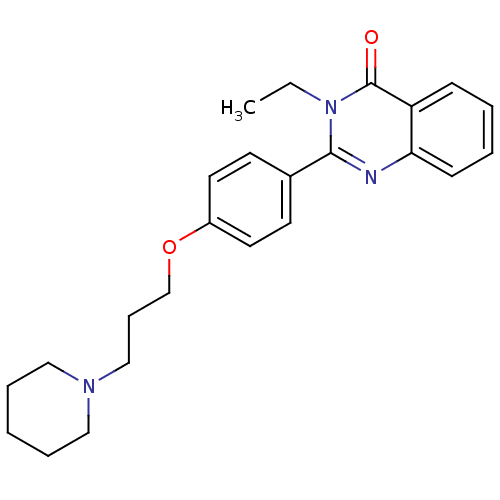
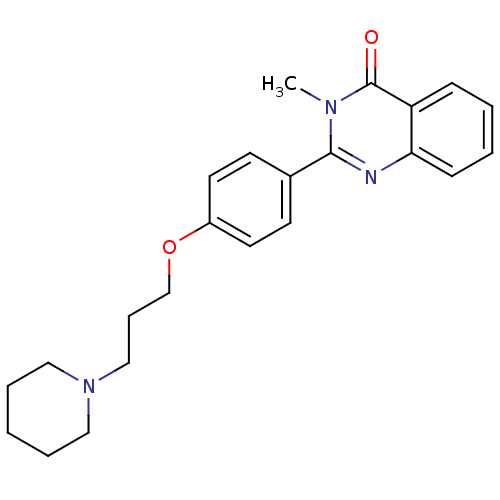
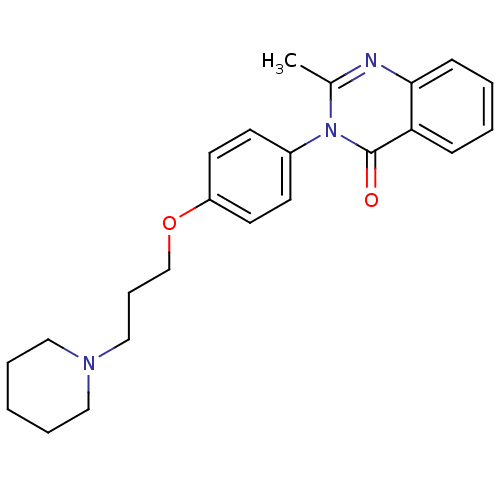
Report error Found 60 Enz. Inhib. hit(s) with all data for entry = 50027495
Affinity DataIC50: 0.100nMAssay Description:Inverse agonist activity at human histamine H3 receptor assessed as inhibition of R-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 0.180nMAssay Description:Inverse agonist activity at human histamine H3 receptor assessed as inhibition of R-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 0.220nMAssay Description:Inverse agonist activity at human histamine H3 receptor assessed as inhibition of R-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 0.25nMAssay Description:Inverse agonist activity at human histamine H3 receptor assessed as inhibition of R-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 0.310nMAssay Description:Inverse agonist activity at human histamine H3 receptor assessed as inhibition of R-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 0.310nMAssay Description:Inverse agonist activity at human histamine H3 receptor assessed as inhibition of R-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 0.310nMAssay Description:Inverse agonist activity at human histamine H3 receptor assessed as inhibition of R-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 0.340nMAssay Description:Inverse agonist activity at human histamine H3 receptor assessed as inhibition of R-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 0.470nMAssay Description:Inverse agonist activity at human histamine H3 receptor assessed as inhibition of R-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 0.480nMAssay Description:Inverse agonist activity at human histamine H3 receptor assessed as inhibition of R-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 0.540nMAssay Description:Inverse agonist activity at human histamine H3 receptor assessed as inhibition of R-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 0.680nMAssay Description:Inverse agonist activity at human histamine H3 receptor assessed as inhibition of R-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:Inverse agonist activity at human histamine H3 receptor assessed as inhibition of R-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 0.740nMAssay Description:Inverse agonist activity at human histamine H3 receptor assessed as inhibition of R-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 0.870nMAssay Description:Inverse agonist activity at human histamine H3 receptor assessed as inhibition of R-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 0.870nMAssay Description:Inverse agonist activity at human histamine H3 receptor assessed as inhibition of R-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Inverse agonist activity at human histamine H3 receptor assessed as inhibition of R-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMAssay Description:Inverse agonist activity at human histamine H3 receptor assessed as inhibition of R-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 3.10nMAssay Description:Inverse agonist activity at human histamine H3 receptor assessed as inhibition of R-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Tsukuba Research Institute
Curated by ChEMBL
Tsukuba Research Institute
Curated by ChEMBL
Affinity DataIC50: 170nMAssay Description:Displacement of [35S]MK499 from human ERG expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Tsukuba Research Institute
Curated by ChEMBL
Tsukuba Research Institute
Curated by ChEMBL
Affinity DataIC50: 350nMAssay Description:Displacement of [35S]MK499 from human ERG expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Tsukuba Research Institute
Curated by ChEMBL
Tsukuba Research Institute
Curated by ChEMBL
Affinity DataIC50: 460nMAssay Description:Displacement of [35S]MK499 from human ERG expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+3nMAssay Description:Displacement of [3H]prazosin from human adrenergic alpha1A receptor expressed in LMtk- cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Tsukuba Research Institute
Curated by ChEMBL
Tsukuba Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.10E+3nMAssay Description:Displacement of [35S]MK499 from human ERG expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+3nMAssay Description:Displacement of [3H]prazosin from human adrenergic alpha1A receptor expressed in LMtk- cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Tsukuba Research Institute
Curated by ChEMBL
Tsukuba Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.70E+3nMAssay Description:Displacement of [35S]MK499 from human ERG expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.80E+3nMAssay Description:Displacement of [3H]prazosin from human adrenergic alpha1A receptor expressed in LMtk- cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Tsukuba Research Institute
Curated by ChEMBL
Tsukuba Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.80E+3nMAssay Description:Displacement of [35S]MK499 from human ERG expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Tsukuba Research Institute
Curated by ChEMBL
Tsukuba Research Institute
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Displacement of [35S]MK499 from human ERG expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 2.20E+3nMAssay Description:Displacement of [3H]prazosin from human adrenergic alpha1A receptor expressed in LMtk- cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 2.40E+3nMAssay Description:Displacement of [3H]prazosin from human adrenergic alpha1A receptor expressed in LMtk- cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Tsukuba Research Institute
Curated by ChEMBL
Tsukuba Research Institute
Curated by ChEMBL
Affinity DataIC50: 2.80E+3nMAssay Description:Displacement of [35S]MK499 from human ERG expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Tsukuba Research Institute
Curated by ChEMBL
Tsukuba Research Institute
Curated by ChEMBL
Affinity DataIC50: 2.90E+3nMAssay Description:Displacement of [35S]MK499 from human ERG expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Tsukuba Research Institute
Curated by ChEMBL
Tsukuba Research Institute
Curated by ChEMBL
Affinity DataIC50: 2.90E+3nMAssay Description:Displacement of [35S]MK499 from human ERG expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 2.90E+3nMAssay Description:Displacement of [3H]prazosin from human adrenergic alpha1A receptor expressed in LMtk- cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Tsukuba Research Institute
Curated by ChEMBL
Tsukuba Research Institute
Curated by ChEMBL
Affinity DataIC50: 3.00E+3nMAssay Description:Displacement of [35S]MK499 from human ERG expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Tsukuba Research Institute
Curated by ChEMBL
Tsukuba Research Institute
Curated by ChEMBL
Affinity DataIC50: 3.80E+3nMAssay Description:Displacement of [35S]MK499 from human ERG expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 4.20E+3nMAssay Description:Displacement of [3H]prazosin from human adrenergic alpha1A receptor expressed in LMtk- cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Tsukuba Research Institute
Curated by ChEMBL
Tsukuba Research Institute
Curated by ChEMBL
Affinity DataIC50: 4.30E+3nMAssay Description:Displacement of [35S]MK499 from human ERG expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 4.50E+3nMAssay Description:Displacement of [3H]prazosin from human adrenergic alpha1A receptor expressed in LMtk- cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Tsukuba Research Institute
Curated by ChEMBL
Tsukuba Research Institute
Curated by ChEMBL
Affinity DataIC50: 5.60E+3nMAssay Description:Displacement of [35S]MK499 from human ERG expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 5.90E+3nMAssay Description:Displacement of [3H]prazosin from human adrenergic alpha1A receptor expressed in LMtk- cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 6.20E+3nMAssay Description:Displacement of [3H]prazosin from human adrenergic alpha1A receptor expressed in LMtk- cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 6.30E+3nMAssay Description:Displacement of [3H]prazosin from human adrenergic alpha1A receptor expressed in LMtk- cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 6.50E+3nMAssay Description:Displacement of [3H]prazosin from human adrenergic alpha1A receptor expressed in LMtk- cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 6.50E+3nMAssay Description:Displacement of [3H]prazosin from human adrenergic alpha1A receptor expressed in LMtk- cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 7.20E+3nMAssay Description:Displacement of [3H]prazosin from human adrenergic alpha1A receptor expressed in LMtk- cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Tsukuba Research Institute
Curated by ChEMBL
Tsukuba Research Institute
Curated by ChEMBL
Affinity DataIC50: 7.90E+3nMAssay Description:Displacement of [35S]MK499 from human ERG expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Tsukuba Research Institute
Curated by ChEMBL
Tsukuba Research Institute
Curated by ChEMBL
Affinity DataIC50: 7.90E+3nMAssay Description:Displacement of [35S]MK499 from human ERG expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human histamine H2 receptorMore data for this Ligand-Target Pair