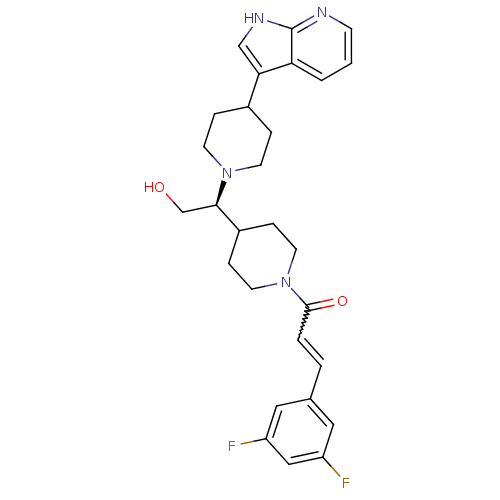
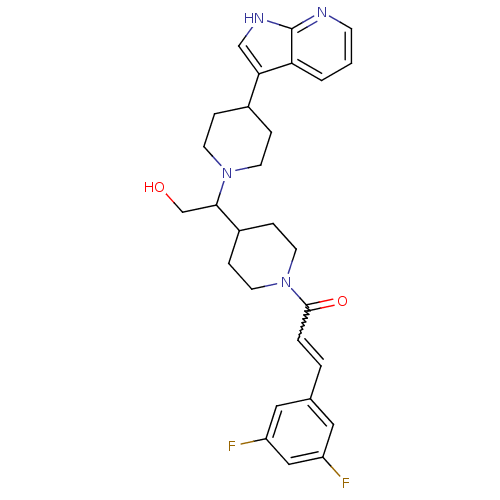
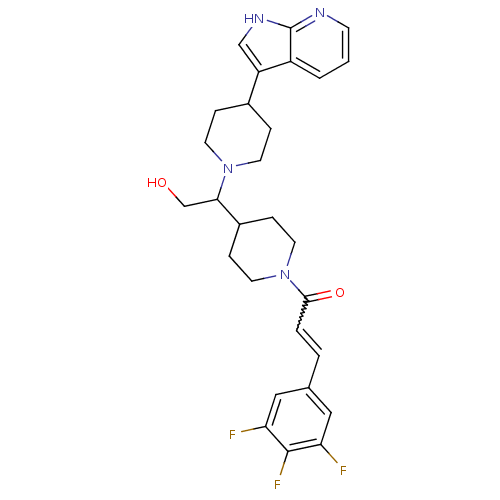
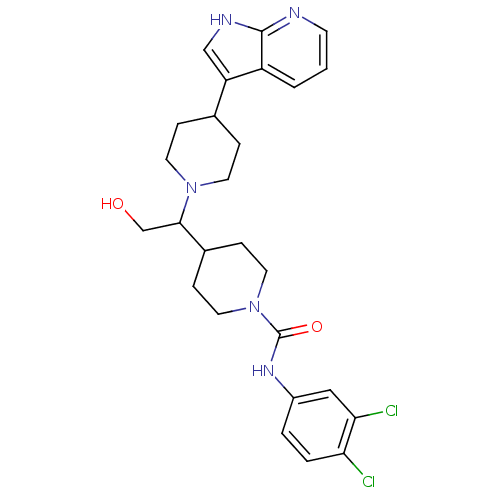
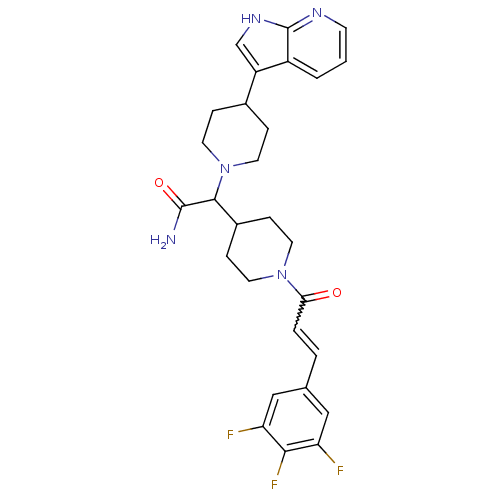
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Antagonist activity at human CCR2More data for this Ligand-Target Pair
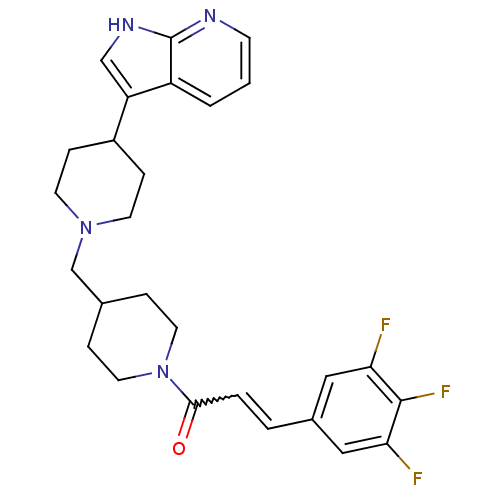
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Antagonist activity at human CCR2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Antagonist activity at human CCR2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 7nMAssay Description:Antagonist activity at human CCR2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 14nMAssay Description:Antagonist activity at human CCR2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 17nMAssay Description:Antagonist activity at human CCR2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 960nMAssay Description:Antagonist activity at human CCR2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 1.30E+3nMAssay Description:Antagonist activity at human CCR2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 1.90E+3nMAssay Description:Antagonist activity at human CCR2More data for this Ligand-Target Pair
TargetCytochrome P450 2D6(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 1.33E+4nMAssay Description:Inhibition of human CYP2D6More data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 2.06E+4nMAssay Description:Inhibition of human CYP3A4 using dibenzylfluorescein as substrateMore data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 2.52E+4nMAssay Description:Inhibition of human CYP3A4 using 7-benzyloxy-4-trifluoromethylcoumarin as substrateMore data for this Ligand-Target Pair
TargetCytochrome P450 2C19(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 2.71E+4nMAssay Description:Inhibition of human CYP2C19More data for this Ligand-Target Pair
TargetCytochrome P450 1A2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: >4.00E+4nMAssay Description:Inhibition of human CYP1A2More data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: >4.00E+4nMAssay Description:Inhibition of human CYP3A4 using 7-benzyloxyquinoline as substrateMore data for this Ligand-Target Pair