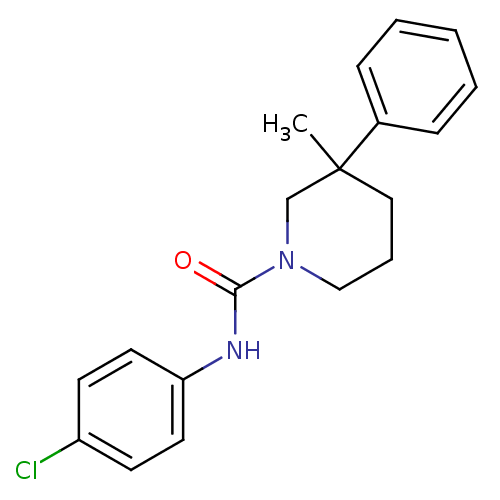
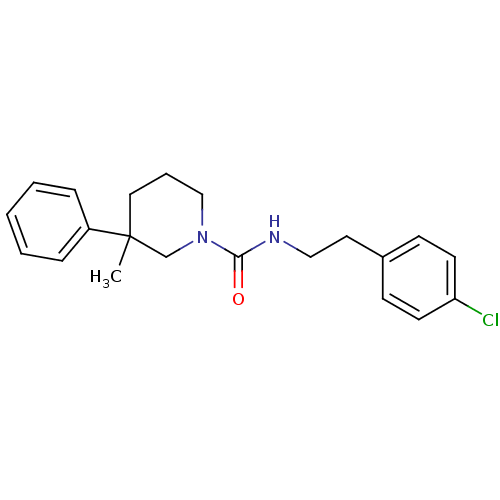
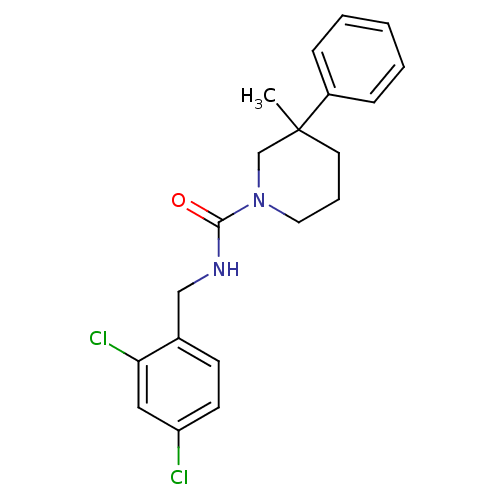
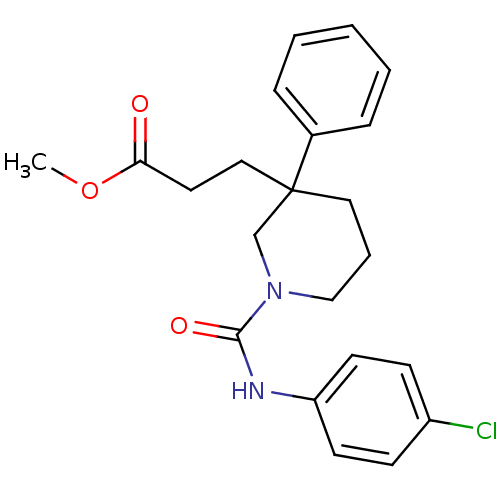
Report error Found 211 Enz. Inhib. hit(s) with all data for entry = 50031304
Affinity DataIC50: 1nMAssay Description:Inhibition of human soluble epoxide hydrolaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human soluble epoxide hydrolaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human soluble epoxide hydrolaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human soluble epoxide hydrolaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human soluble epoxide hydrolaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:Inhibition of human soluble epoxide hydrolaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:Inhibition of human soluble epoxide hydrolaseMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of rat soluble epoxide hydrolaseMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of rat soluble epoxide hydrolaseMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of human soluble epoxide hydrolaseMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of human soluble epoxide hydrolaseMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of rat soluble epoxide hydrolaseMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of rat soluble epoxide hydrolaseMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of rat soluble epoxide hydrolaseMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of human soluble epoxide hydrolaseMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of human soluble epoxide hydrolaseMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of human soluble epoxide hydrolaseMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of human soluble epoxide hydrolaseMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of human soluble epoxide hydrolaseMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of human soluble epoxide hydrolaseMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of human soluble epoxide hydrolaseMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of rat soluble epoxide hydrolaseMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of rat soluble epoxide hydrolaseMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of rat soluble epoxide hydrolaseMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of rat soluble epoxide hydrolaseMore data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Inhibition of human soluble epoxide hydrolaseMore data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Inhibition of rat soluble epoxide hydrolaseMore data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Inhibition of human soluble epoxide hydrolaseMore data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Inhibition of human soluble epoxide hydrolaseMore data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Inhibition of human soluble epoxide hydrolaseMore data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Inhibition of human soluble epoxide hydrolaseMore data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Inhibition of human soluble epoxide hydrolaseMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of rat soluble epoxide hydrolaseMore data for this Ligand-Target Pair
Affinity DataIC50: 11nMAssay Description:Inhibition of rat soluble epoxide hydrolaseMore data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:Inhibition of human soluble epoxide hydrolaseMore data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:Inhibition of human soluble epoxide hydrolaseMore data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:Inhibition of human soluble epoxide hydrolaseMore data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:Inhibition of human soluble epoxide hydrolaseMore data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:Inhibition of rat soluble epoxide hydrolaseMore data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Inhibition of human soluble epoxide hydrolaseMore data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Inhibition of rat soluble epoxide hydrolaseMore data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:Inhibition of rat soluble epoxide hydrolaseMore data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:Inhibition of human soluble epoxide hydrolaseMore data for this Ligand-Target Pair
Affinity DataIC50: 26nMAssay Description:Inhibition of rat soluble epoxide hydrolaseMore data for this Ligand-Target Pair
Affinity DataIC50: 26nMAssay Description:Inhibition of rat soluble epoxide hydrolaseMore data for this Ligand-Target Pair
Affinity DataIC50: 28nMAssay Description:Inhibition of human soluble epoxide hydrolaseMore data for this Ligand-Target Pair
Affinity DataIC50: 29nMAssay Description:Inhibition of human soluble epoxide hydrolaseMore data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibition of human soluble epoxide hydrolaseMore data for this Ligand-Target Pair
Affinity DataIC50: 31nMAssay Description:Inhibition of rat soluble epoxide hydrolaseMore data for this Ligand-Target Pair
Affinity DataIC50: 32nMAssay Description:Inhibition of rat soluble epoxide hydrolaseMore data for this Ligand-Target Pair