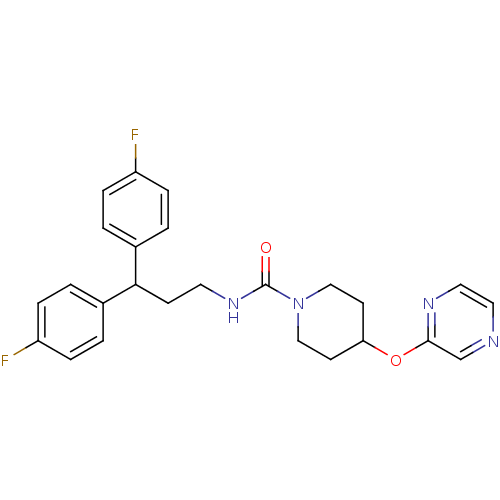
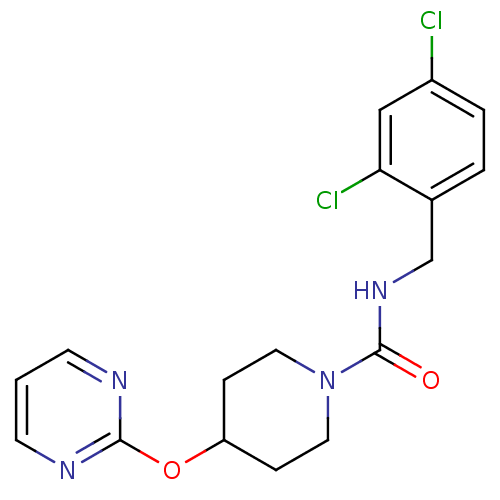
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of human soluble epoxide hydrolaseMore data for this Ligand-Target Pair
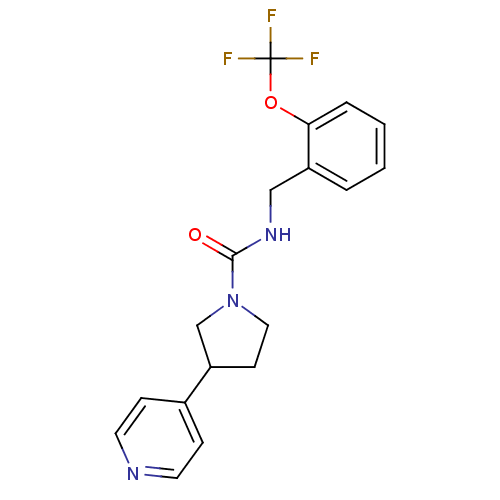
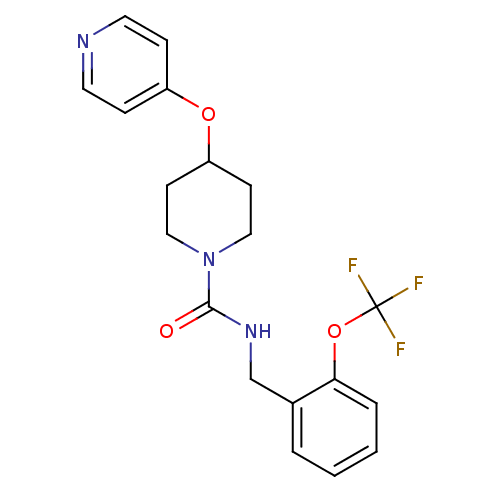
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of human soluble epoxide hydrolaseMore data for this Ligand-Target Pair
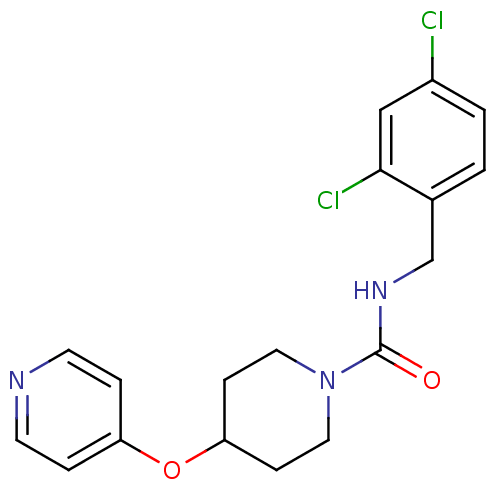
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of human soluble epoxide hydrolase assessed as [2-3H]-trans-1,3-diphenyl propylene oxide hydrolysis by cellular assayMore data for this Ligand-Target Pair
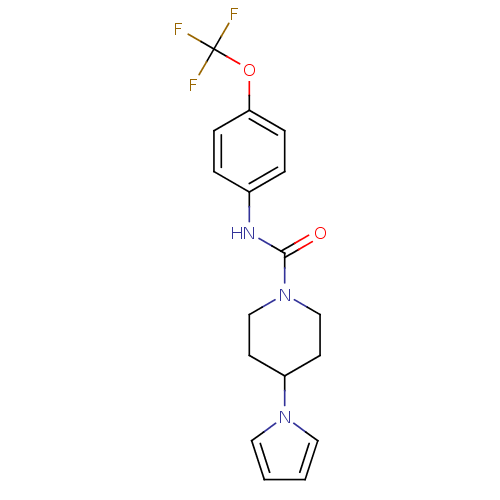
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of human soluble epoxide hydrolaseMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 7nMAssay Description:Inhibition of human soluble epoxide hydrolaseMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 9nMAssay Description:Inhibition of human soluble epoxide hydrolaseMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Rattus norvegicus)
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 11nMAssay Description:Inhibition of rat soluble epoxide hydrolaseMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Rattus norvegicus)
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 11nMAssay Description:Inhibition of rat soluble epoxide hydrolaseMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 12nMAssay Description:Inhibition of human soluble epoxide hydrolaseMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Inhibition of human soluble epoxide hydrolase assessed as [2-3H]-trans-1,3-diphenyl propylene oxide hydrolysis by cellular assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 14nMAssay Description:Inhibition of human soluble epoxide hydrolaseMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 18nMAssay Description:Inhibition of human soluble epoxide hydrolaseMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 22nMAssay Description:Inhibition of human soluble epoxide hydrolase assessed as [2-3H]-trans-1,3-diphenyl propylene oxide hydrolysis by cellular assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 40nMAssay Description:Inhibition of human soluble epoxide hydrolase assessed as [2-3H]-trans-1,3-diphenyl propylene oxide hydrolysis by cellular assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 63nMAssay Description:Inhibition of human soluble epoxide hydrolase assessed as [2-3H]-trans-1,3-diphenyl propylene oxide hydrolysis by cellular assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 76nMAssay Description:Inhibition of human soluble epoxide hydrolase assessed as [2-3H]-trans-1,3-diphenyl propylene oxide hydrolysis by cellular assayMore data for this Ligand-Target Pair
TargetCytochrome P450 2C9(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 126nMAssay Description:Inhibition of human soluble epoxide hydrolase assessed as [2-3H]-trans-1,3-diphenyl propylene oxide hydrolysis by cellular assayMore data for this Ligand-Target Pair
TargetCytochrome P450 2C9(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
TargetCytochrome P450 2C9(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
TargetCytochrome P450 2J2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of CYP2J2More data for this Ligand-Target Pair
TargetCytochrome P450 2J2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 2.70E+3nMAssay Description:Inhibition of CYP2J2More data for this Ligand-Target Pair
TargetCytochrome P450 2C9(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 2.90E+3nMAssay Description:Inhibition of CYP2C9More data for this Ligand-Target Pair
TargetCytochrome P450 2C9(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 8.10E+3nMAssay Description:Inhibition of CYP2C9More data for this Ligand-Target Pair
TargetCytochrome P450 2J2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of CYP2J2More data for this Ligand-Target Pair
TargetCytochrome P450 2J2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of CYP2J2More data for this Ligand-Target Pair
TargetCytochrome P450 2C9(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 1.02E+4nMAssay Description:Inhibition of CYP2C9More data for this Ligand-Target Pair
TargetCytochrome P450 2J2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of CYP2J2More data for this Ligand-Target Pair
TargetCytochrome P450 2C9(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 2.55E+4nMAssay Description:Inhibition of CYP2C9More data for this Ligand-Target Pair



 3D Structure (crystal)
3D Structure (crystal)