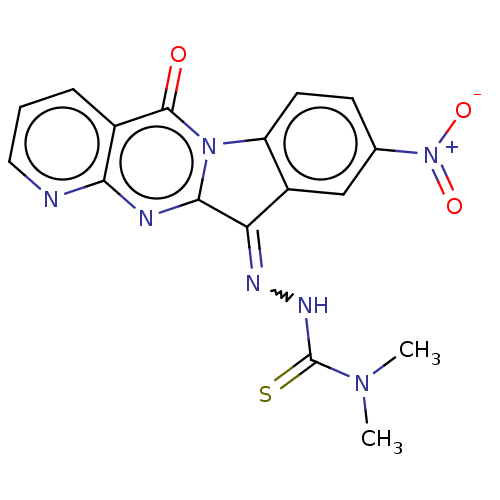
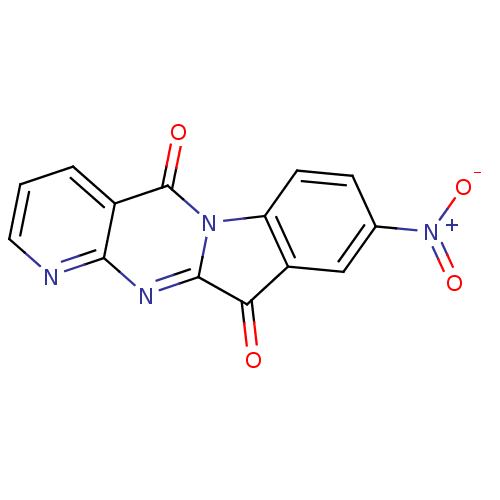
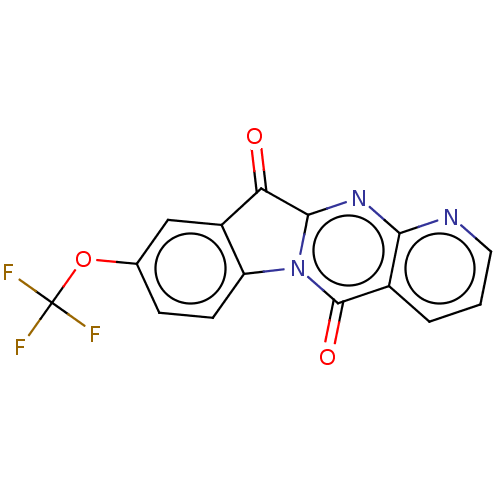
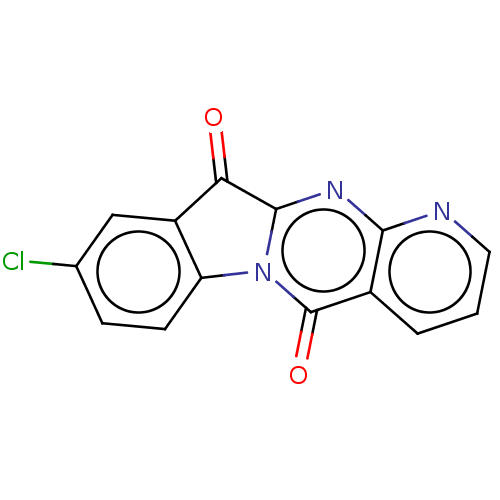
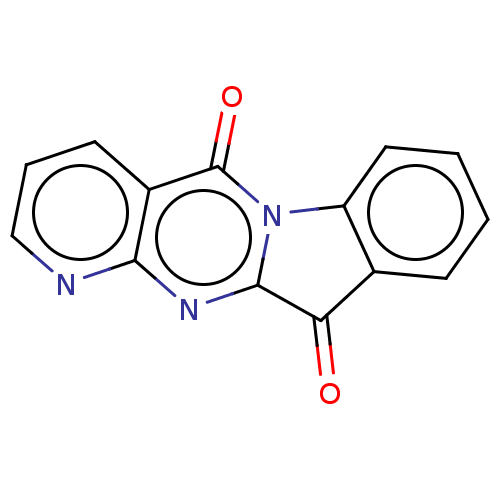
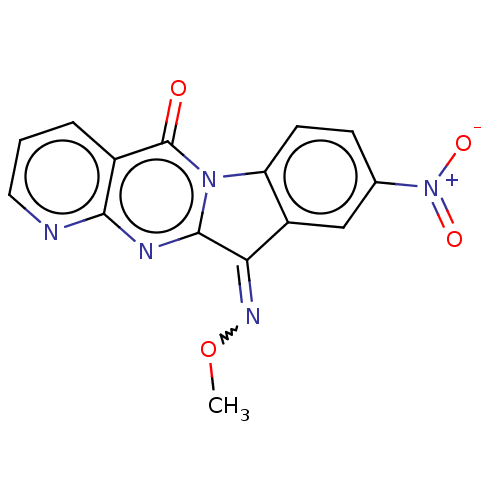
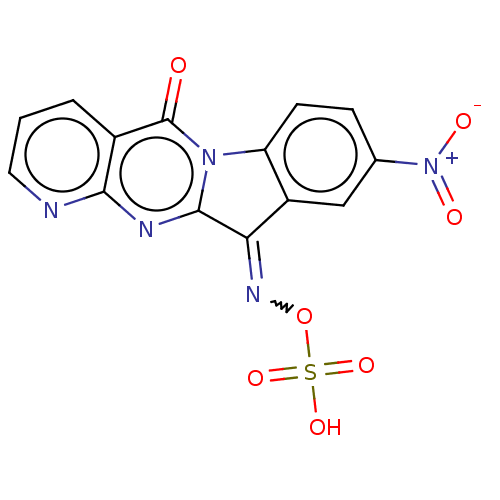
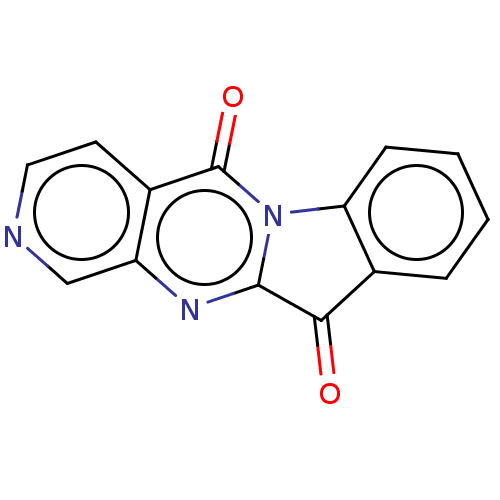
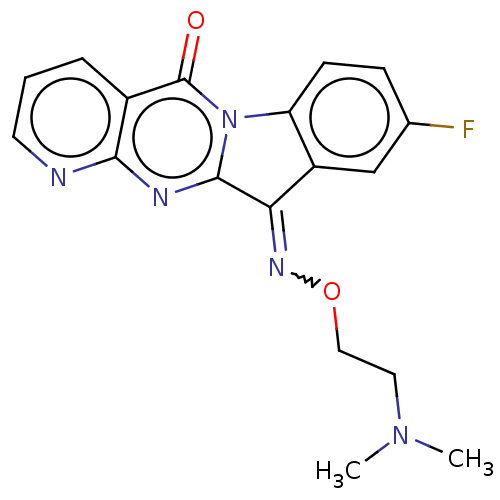
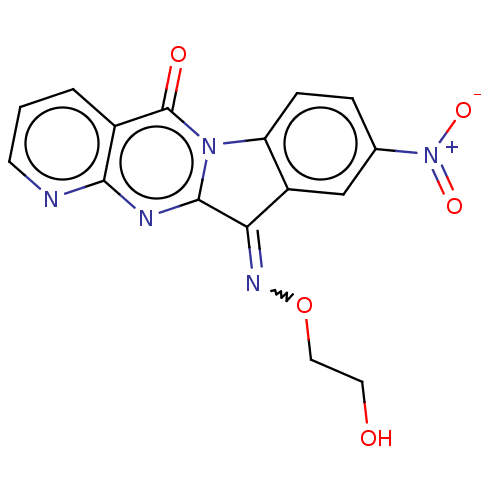
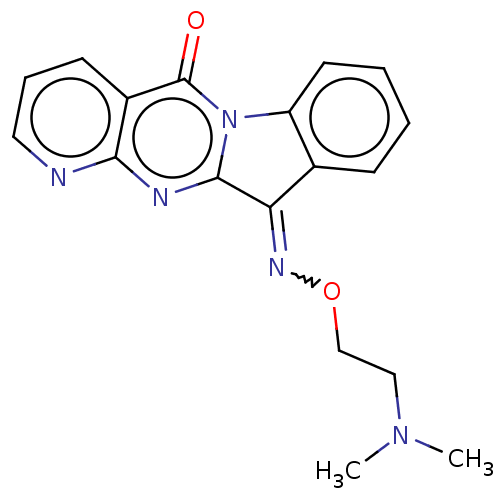
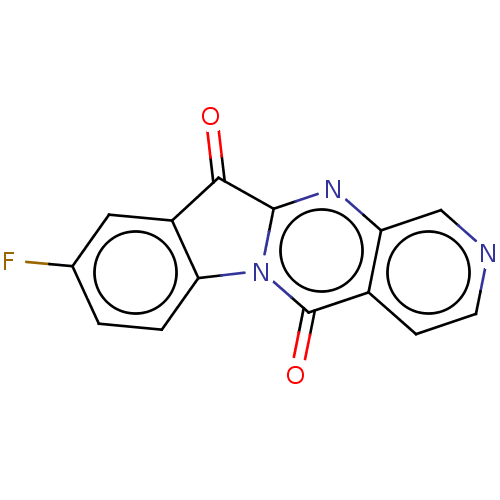
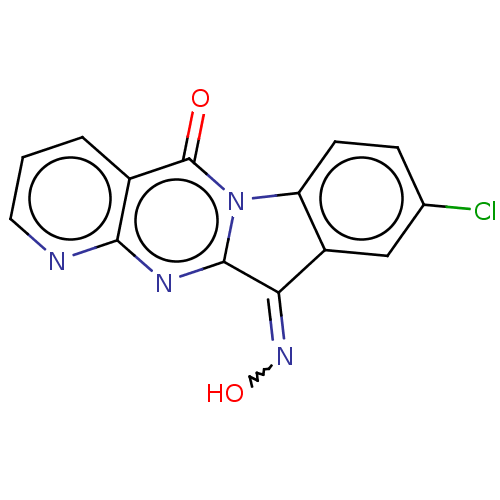
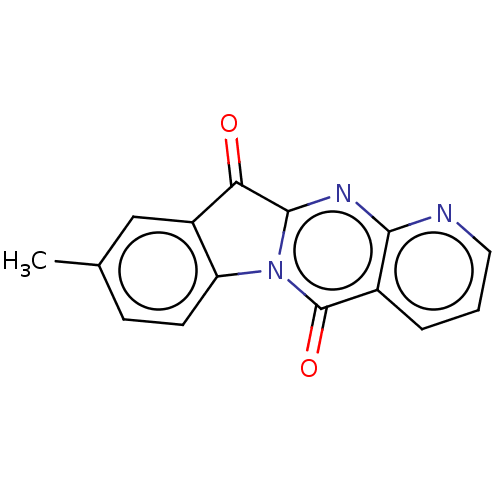
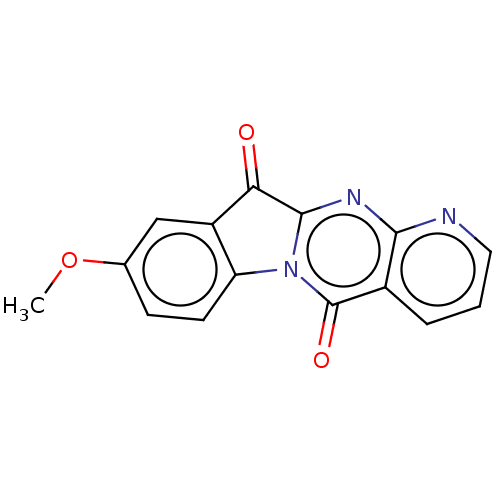
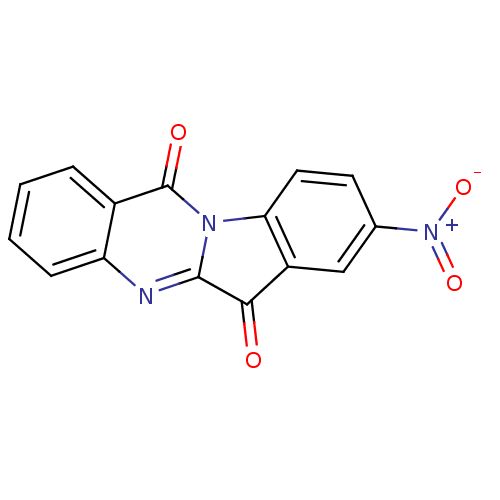
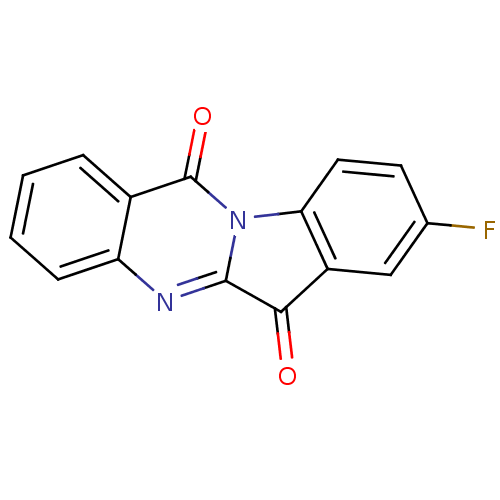
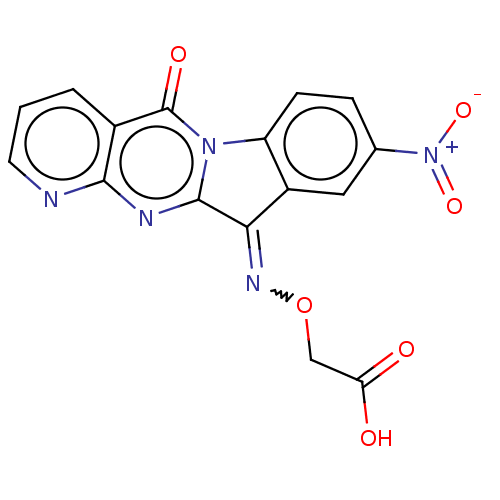
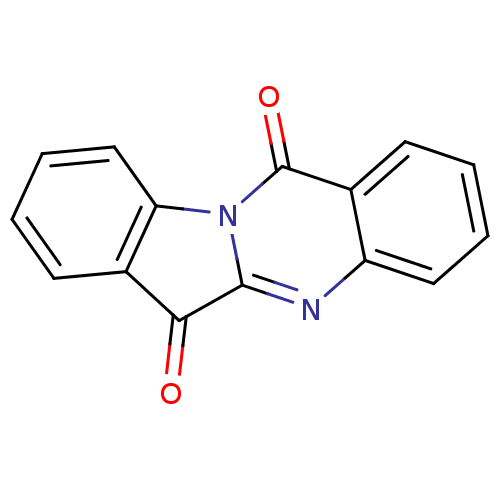
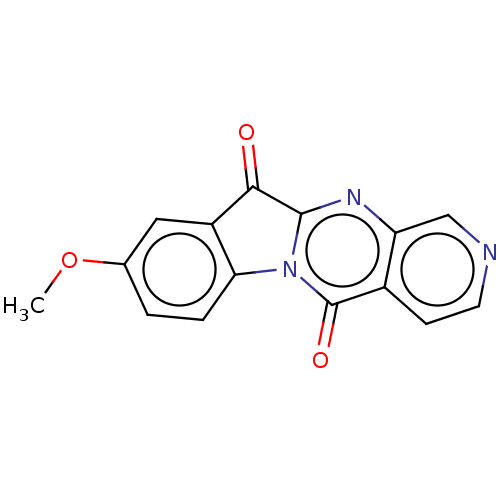
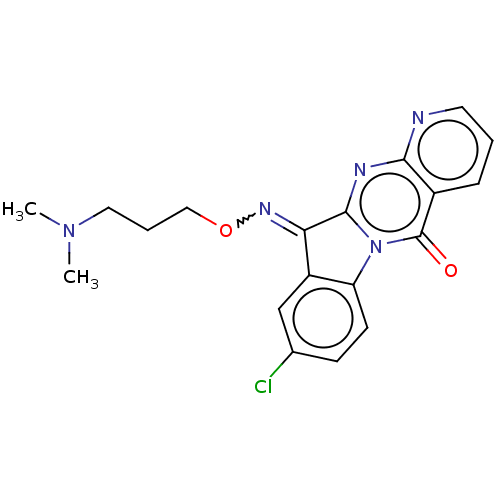
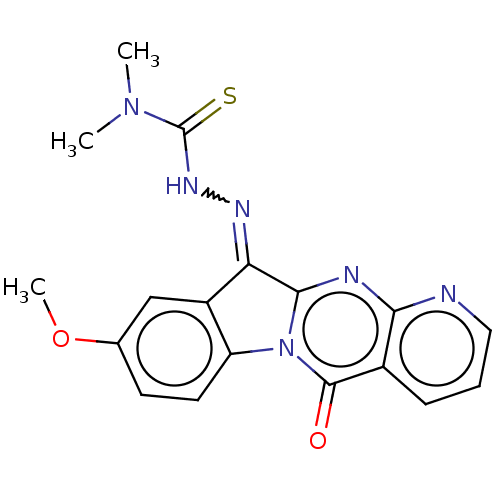
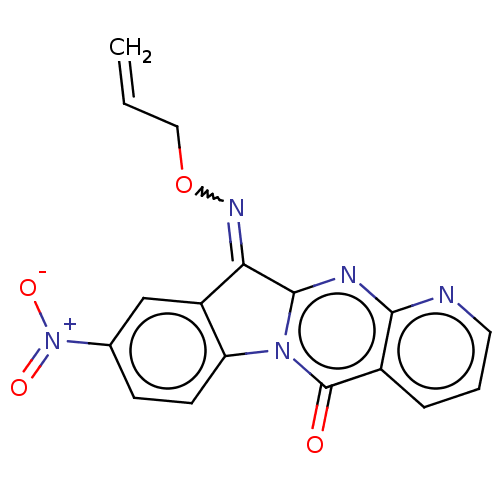
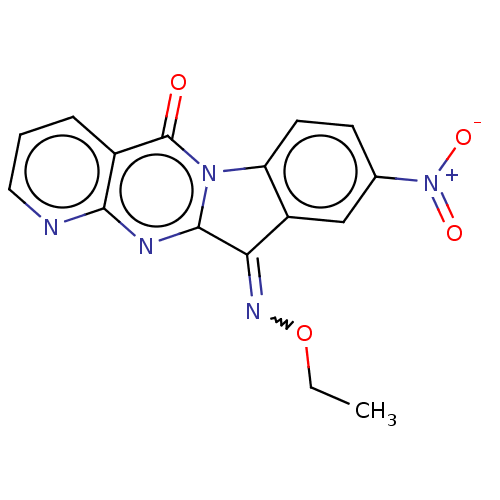
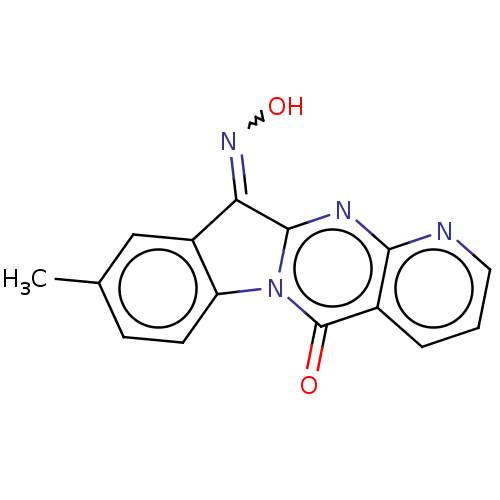
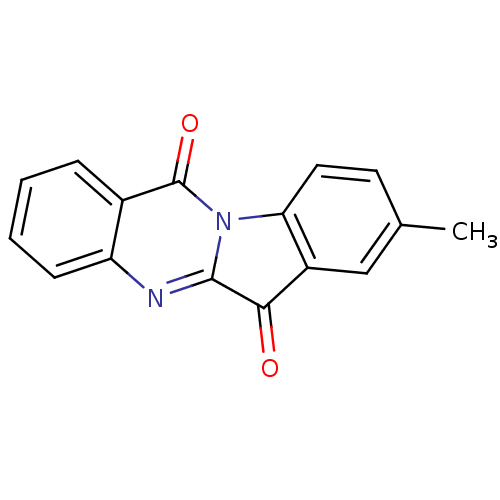
Report error Found 42 Enz. Inhib. hit(s) with all data for entry = 9166
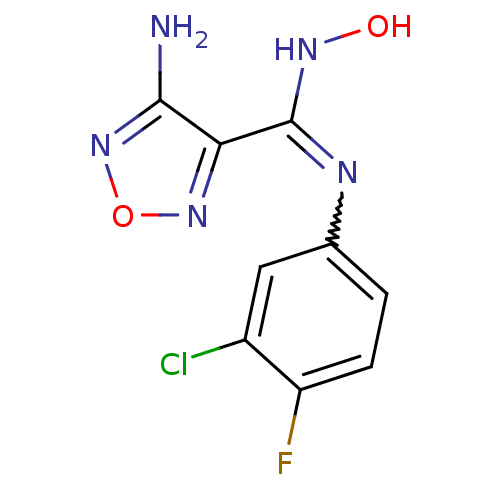
Affinity DataIC50: 5.5nMAssay Description:Experimental method: IDO-1 can oxidatively cleave the indole ring of tryptophan to form N-formylkynurenine. Referring to the method in the literature...More data for this Ligand-Target Pair
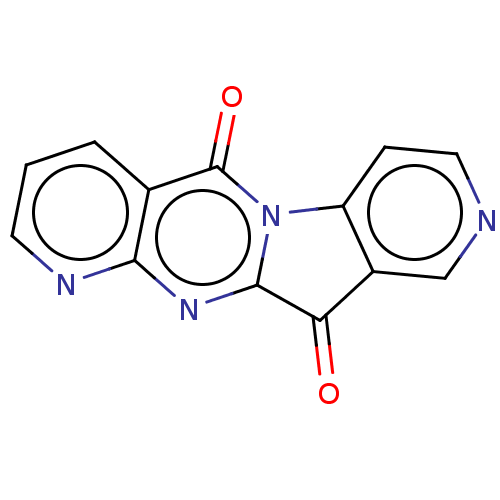
Affinity DataIC50: 5.5nMAssay Description:Experimental method: IDO-1 can oxidatively cleave the indole ring of tryptophan to form N-formylkynurenine. Referring to the method in the literature...More data for this Ligand-Target Pair
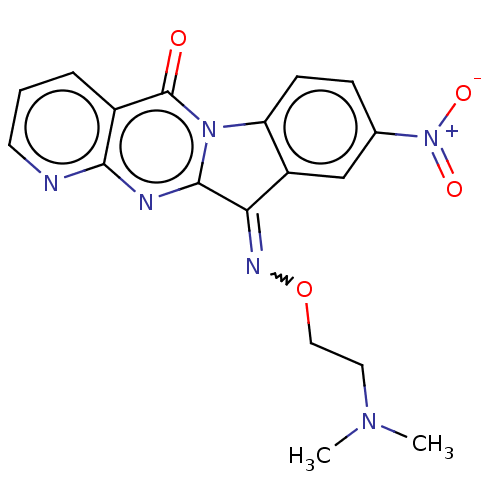
Affinity DataIC50: 5.5nMAssay Description:Experimental method: IDO-1 can oxidatively cleave the indole ring of tryptophan to form N-formylkynurenine. Referring to the method in the literature...More data for this Ligand-Target Pair
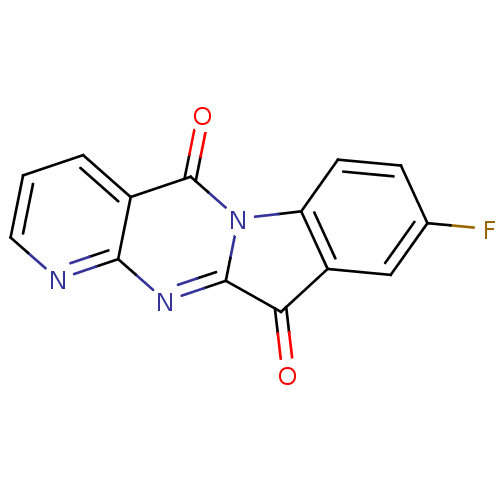
Affinity DataIC50: 5.5nMAssay Description:Experimental method: IDO-1 can oxidatively cleave the indole ring of tryptophan to form N-formylkynurenine. Referring to the method in the literature...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Experimental method: IDO-1 can oxidatively cleave the indole ring of tryptophan to form N-formylkynurenine. Referring to the method in the literature...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Experimental method: IDO-1 can oxidatively cleave the indole ring of tryptophan to form N-formylkynurenine. Referring to the method in the literature...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Experimental method: IDO-1 can oxidatively cleave the indole ring of tryptophan to form N-formylkynurenine. Referring to the method in the literature...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Experimental method: IDO-1 can oxidatively cleave the indole ring of tryptophan to form N-formylkynurenine. Referring to the method in the literature...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Experimental method: IDO-1 can oxidatively cleave the indole ring of tryptophan to form N-formylkynurenine. Referring to the method in the literature...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Experimental method: IDO-1 can oxidatively cleave the indole ring of tryptophan to form N-formylkynurenine. Referring to the method in the literature...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Experimental method: IDO-1 can oxidatively cleave the indole ring of tryptophan to form N-formylkynurenine. Referring to the method in the literature...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Experimental method: IDO-1 can oxidatively cleave the indole ring of tryptophan to form N-formylkynurenine. Referring to the method in the literature...More data for this Ligand-Target Pair
Affinity DataIC50: 550nMAssay Description:Experimental method: IDO-1 can oxidatively cleave the indole ring of tryptophan to form N-formylkynurenine. Referring to the method in the literature...More data for this Ligand-Target Pair
Affinity DataIC50: 550nMAssay Description:Experimental method: IDO-1 can oxidatively cleave the indole ring of tryptophan to form N-formylkynurenine. Referring to the method in the literature...More data for this Ligand-Target Pair
Affinity DataIC50: 550nMAssay Description:Experimental method: IDO-1 can oxidatively cleave the indole ring of tryptophan to form N-formylkynurenine. Referring to the method in the literature...More data for this Ligand-Target Pair
Affinity DataIC50: 550nMAssay Description:Experimental method: IDO-1 can oxidatively cleave the indole ring of tryptophan to form N-formylkynurenine. Referring to the method in the literature...More data for this Ligand-Target Pair
Affinity DataIC50: 550nMAssay Description:Experimental method: IDO-1 can oxidatively cleave the indole ring of tryptophan to form N-formylkynurenine. Referring to the method in the literature...More data for this Ligand-Target Pair
Affinity DataIC50: 550nMAssay Description:Experimental method: IDO-1 can oxidatively cleave the indole ring of tryptophan to form N-formylkynurenine. Referring to the method in the literature...More data for this Ligand-Target Pair
Affinity DataIC50: 550nMAssay Description:Experimental method: IDO-1 can oxidatively cleave the indole ring of tryptophan to form N-formylkynurenine. Referring to the method in the literature...More data for this Ligand-Target Pair
Affinity DataIC50: 550nMAssay Description:Experimental method: IDO-1 can oxidatively cleave the indole ring of tryptophan to form N-formylkynurenine. Referring to the method in the literature...More data for this Ligand-Target Pair
Affinity DataIC50: 550nMAssay Description:Experimental method: IDO-1 can oxidatively cleave the indole ring of tryptophan to form N-formylkynurenine. Referring to the method in the literature...More data for this Ligand-Target Pair
Affinity DataIC50: 550nMAssay Description:Experimental method: IDO-1 can oxidatively cleave the indole ring of tryptophan to form N-formylkynurenine. Referring to the method in the literature...More data for this Ligand-Target Pair
Affinity DataIC50: 550nMAssay Description:Experimental method: IDO-1 can oxidatively cleave the indole ring of tryptophan to form N-formylkynurenine. Referring to the method in the literature...More data for this Ligand-Target Pair
Affinity DataIC50: 550nMAssay Description:Experimental method: IDO-1 can oxidatively cleave the indole ring of tryptophan to form N-formylkynurenine. Referring to the method in the literature...More data for this Ligand-Target Pair
Affinity DataIC50: 550nMAssay Description:Experimental method: IDO-1 can oxidatively cleave the indole ring of tryptophan to form N-formylkynurenine. Referring to the method in the literature...More data for this Ligand-Target Pair
Affinity DataIC50: 550nMAssay Description:Experimental method: IDO-1 can oxidatively cleave the indole ring of tryptophan to form N-formylkynurenine. Referring to the method in the literature...More data for this Ligand-Target Pair
Affinity DataIC50: 550nMAssay Description:Experimental method: IDO-1 can oxidatively cleave the indole ring of tryptophan to form N-formylkynurenine. Referring to the method in the literature...More data for this Ligand-Target Pair
Affinity DataIC50: 550nMAssay Description:Experimental method: IDO-1 can oxidatively cleave the indole ring of tryptophan to form N-formylkynurenine. Referring to the method in the literature...More data for this Ligand-Target Pair
Affinity DataIC50: 550nMAssay Description:Experimental method: IDO-1 can oxidatively cleave the indole ring of tryptophan to form N-formylkynurenine. Referring to the method in the literature...More data for this Ligand-Target Pair
Affinity DataIC50: 550nMAssay Description:Experimental method: IDO-1 can oxidatively cleave the indole ring of tryptophan to form N-formylkynurenine. Referring to the method in the literature...More data for this Ligand-Target Pair
Affinity DataIC50: 550nMAssay Description:Experimental method: IDO-1 can oxidatively cleave the indole ring of tryptophan to form N-formylkynurenine. Referring to the method in the literature...More data for this Ligand-Target Pair
Affinity DataIC50: 550nMAssay Description:Experimental method: IDO-1 can oxidatively cleave the indole ring of tryptophan to form N-formylkynurenine. Referring to the method in the literature...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMAssay Description:Experimental method: IDO-1 can oxidatively cleave the indole ring of tryptophan to form N-formylkynurenine. Referring to the method in the literature...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMAssay Description:Experimental method: IDO-1 can oxidatively cleave the indole ring of tryptophan to form N-formylkynurenine. Referring to the method in the literature...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMAssay Description:Experimental method: IDO-1 can oxidatively cleave the indole ring of tryptophan to form N-formylkynurenine. Referring to the method in the literature...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMAssay Description:Experimental method: IDO-1 can oxidatively cleave the indole ring of tryptophan to form N-formylkynurenine. Referring to the method in the literature...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMAssay Description:Experimental method: IDO-1 can oxidatively cleave the indole ring of tryptophan to form N-formylkynurenine. Referring to the method in the literature...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMAssay Description:Experimental method: IDO-1 can oxidatively cleave the indole ring of tryptophan to form N-formylkynurenine. Referring to the method in the literature...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMAssay Description:Experimental method: IDO-1 can oxidatively cleave the indole ring of tryptophan to form N-formylkynurenine. Referring to the method in the literature...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMAssay Description:Experimental method: IDO-1 can oxidatively cleave the indole ring of tryptophan to form N-formylkynurenine. Referring to the method in the literature...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMAssay Description:Experimental method: IDO-1 can oxidatively cleave the indole ring of tryptophan to form N-formylkynurenine. Referring to the method in the literature...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Experimental method: IDO-1 can oxidatively cleave the indole ring of tryptophan to form N-formylkynurenine. Referring to the method in the literature...More data for this Ligand-Target Pair