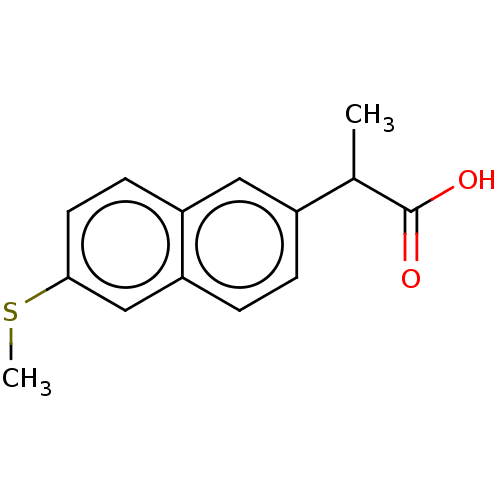
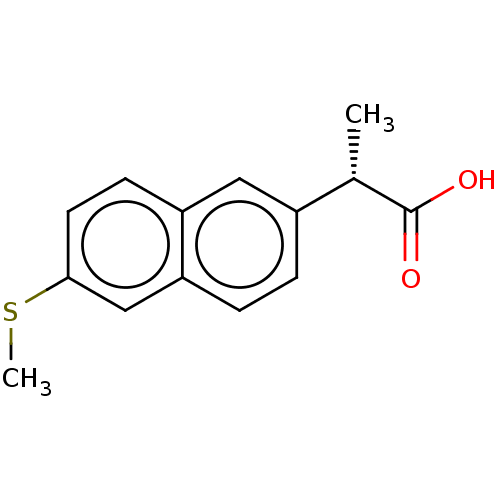
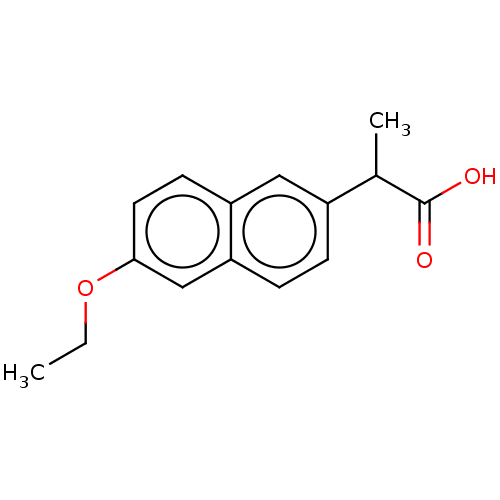
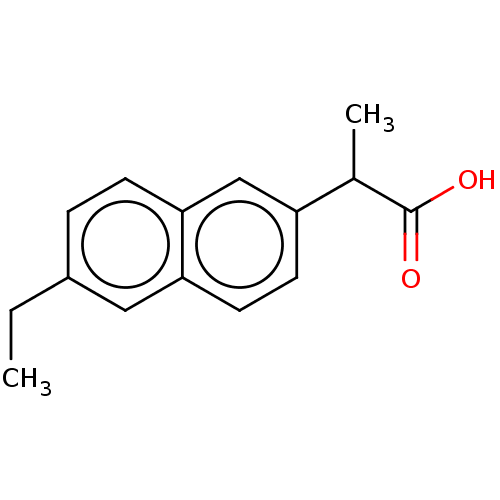
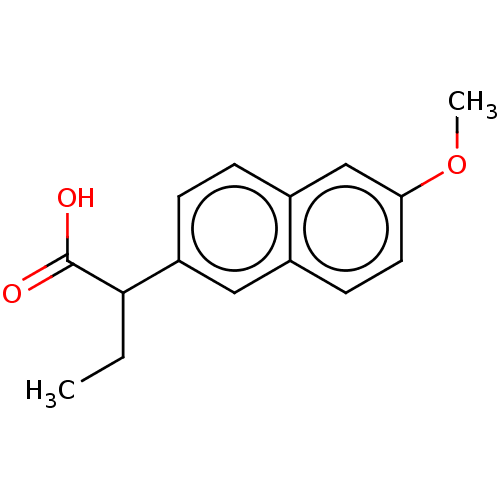
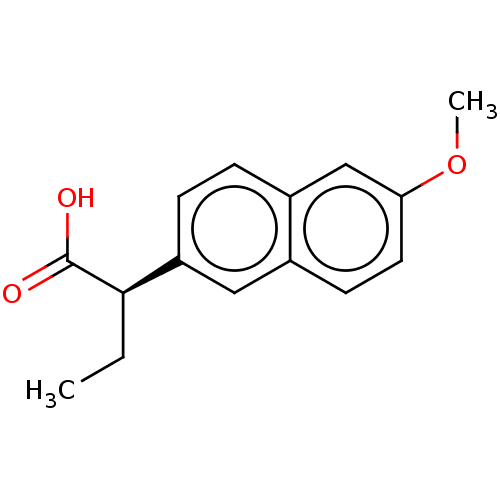
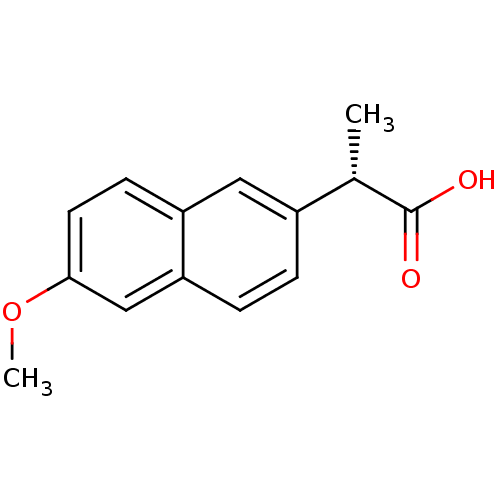
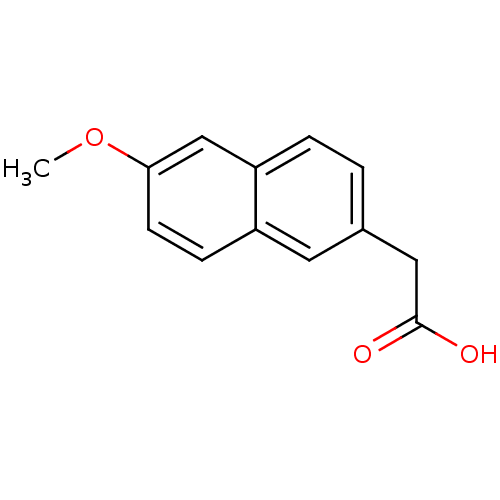
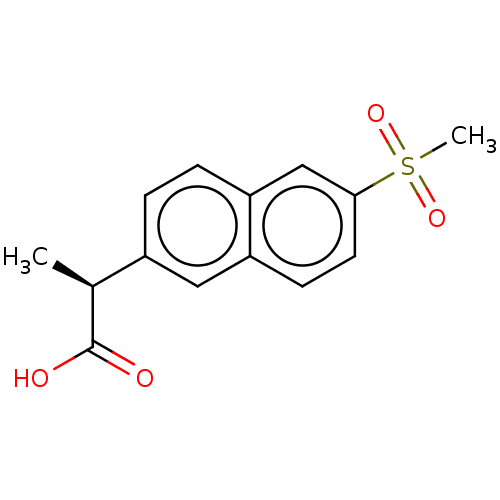
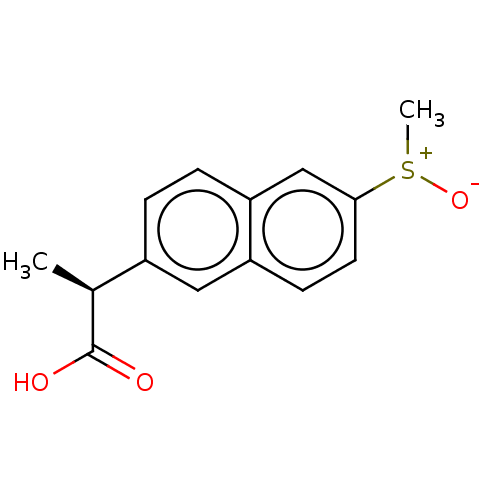
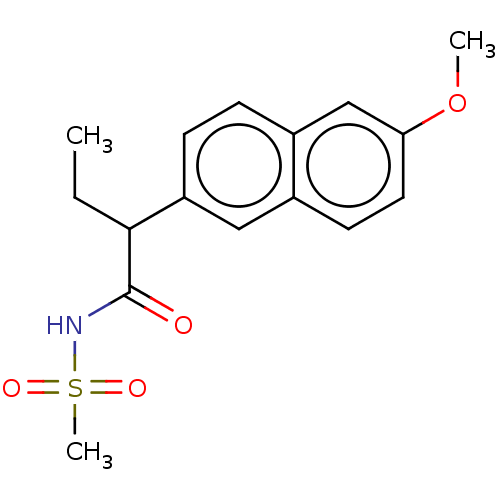
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees Of The University Of Pennsylvania
US Patent
The Trustees Of The University Of Pennsylvania
US Patent
Affinity DataIC50: 50nMAssay Description:the inhibitory potency of the individual compounds against the AKR1C isoforms was determined by monitoring the NADP+ dependent oxidation of S-tetralo...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees Of The University Of Pennsylvania
US Patent
The Trustees Of The University Of Pennsylvania
US Patent
Affinity DataIC50: 50nMAssay Description:the inhibitory potency of the individual compounds against the AKR1C isoforms was determined by monitoring the NADP+ dependent oxidation of S-tetralo...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees Of The University Of Pennsylvania
US Patent
The Trustees Of The University Of Pennsylvania
US Patent
Affinity DataIC50: 50nMAssay Description:the inhibitory potency of the individual compounds against the AKR1C isoforms was determined by monitoring the NADP+ dependent oxidation of S-tetralo...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees Of The University Of Pennsylvania
US Patent
The Trustees Of The University Of Pennsylvania
US Patent
Affinity DataIC50: 60nMAssay Description:the inhibitory potency of the individual compounds against the AKR1C isoforms was determined by monitoring the NADP+ dependent oxidation of S-tetralo...More data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 1(Homo sapiens (Human))
The Trustees Of The University Of Pennsylvania
US Patent
The Trustees Of The University Of Pennsylvania
US Patent
Affinity DataIC50: 61nMAssay Description:The effect of the compounds on COX-1 activity was determined by a continuous colorimetric assay that monitored the oxidation of N, N, N, N-tetramethy...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees Of The University Of Pennsylvania
US Patent
The Trustees Of The University Of Pennsylvania
US Patent
Affinity DataIC50: 70nMAssay Description:the inhibitory potency of the individual compounds against the AKR1C isoforms was determined by monitoring the NADP+ dependent oxidation of S-tetralo...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees Of The University Of Pennsylvania
US Patent
The Trustees Of The University Of Pennsylvania
US Patent
Affinity DataIC50: 100nMAssay Description:the inhibitory potency of the individual compounds against the AKR1C isoforms was determined by monitoring the NADP+ dependent oxidation of S-tetralo...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees Of The University Of Pennsylvania
US Patent
The Trustees Of The University Of Pennsylvania
US Patent
Affinity DataIC50: 110nMAssay Description:the inhibitory potency of the individual compounds against the AKR1C isoforms was determined by monitoring the NADP+ dependent oxidation of S-tetralo...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees Of The University Of Pennsylvania
US Patent
The Trustees Of The University Of Pennsylvania
US Patent
Affinity DataIC50: 110nMAssay Description:the inhibitory potency of the individual compounds against the AKR1C isoforms was determined by monitoring the NADP+ dependent oxidation of S-tetralo...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees Of The University Of Pennsylvania
US Patent
The Trustees Of The University Of Pennsylvania
US Patent
Affinity DataIC50: 120nMAssay Description:the inhibitory potency of the individual compounds against the AKR1C isoforms was determined by monitoring the NADP+ dependent oxidation of S-tetralo...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees Of The University Of Pennsylvania
US Patent
The Trustees Of The University Of Pennsylvania
US Patent
Affinity DataIC50: 120nMAssay Description:the inhibitory potency of the individual compounds against the AKR1C isoforms was determined by monitoring the NADP+ dependent oxidation of S-tetralo...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees Of The University Of Pennsylvania
US Patent
The Trustees Of The University Of Pennsylvania
US Patent
Affinity DataIC50: 120nMAssay Description:the inhibitory potency of the individual compounds against the AKR1C isoforms was determined by monitoring the NADP+ dependent oxidation of S-tetralo...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees Of The University Of Pennsylvania
US Patent
The Trustees Of The University Of Pennsylvania
US Patent
Affinity DataIC50: 120nMAssay Description:the inhibitory potency of the individual compounds against the AKR1C isoforms was determined by monitoring the NADP+ dependent oxidation of S-tetralo...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees Of The University Of Pennsylvania
US Patent
The Trustees Of The University Of Pennsylvania
US Patent
Affinity DataIC50: 180nMAssay Description:the inhibitory potency of the individual compounds against the AKR1C isoforms was determined by monitoring the NADP+ dependent oxidation of S-tetralo...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees Of The University Of Pennsylvania
US Patent
The Trustees Of The University Of Pennsylvania
US Patent
Affinity DataIC50: 180nMAssay Description:the inhibitory potency of the individual compounds against the AKR1C isoforms was determined by monitoring the NADP+ dependent oxidation of S-tetralo...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees Of The University Of Pennsylvania
US Patent
The Trustees Of The University Of Pennsylvania
US Patent
Affinity DataIC50: 270nMAssay Description:the inhibitory potency of the individual compounds against the AKR1C isoforms was determined by monitoring the NADP+ dependent oxidation of S-tetralo...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees Of The University Of Pennsylvania
US Patent
The Trustees Of The University Of Pennsylvania
US Patent
Affinity DataIC50: 650nMAssay Description:the inhibitory potency of the individual compounds against the AKR1C isoforms was determined by monitoring the NADP+ dependent oxidation of S-tetralo...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees Of The University Of Pennsylvania
US Patent
The Trustees Of The University Of Pennsylvania
US Patent
Affinity DataIC50: 820nMAssay Description:the inhibitory potency of the individual compounds against the AKR1C isoforms was determined by monitoring the NADP+ dependent oxidation of S-tetralo...More data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 2(Homo sapiens (Human))
The Trustees Of The University Of Pennsylvania
US Patent
The Trustees Of The University Of Pennsylvania
US Patent
Affinity DataIC50: 900nMAssay Description:The effect of the compounds on COX-1 activity was determined by a continuous colorimetric assay that monitored the oxidation of N, N, N, N-tetramethy...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees Of The University Of Pennsylvania
US Patent
The Trustees Of The University Of Pennsylvania
US Patent
Affinity DataIC50: 1.05E+3nMAssay Description:the inhibitory potency of the individual compounds against the AKR1C isoforms was determined by monitoring the NADP+ dependent oxidation of S-tetralo...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
The Trustees Of The University Of Pennsylvania
US Patent
The Trustees Of The University Of Pennsylvania
US Patent
Affinity DataIC50: 1.26E+3nMAssay Description:the inhibitory potency of the individual compounds against the AKR1C isoforms was determined by monitoring the NADP+ dependent oxidation of S-tetralo...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
The Trustees Of The University Of Pennsylvania
US Patent
The Trustees Of The University Of Pennsylvania
US Patent
Affinity DataIC50: 1.32E+3nMAssay Description:the inhibitory potency of the individual compounds against the AKR1C isoforms was determined by monitoring the NADP+ dependent oxidation of S-tetralo...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
The Trustees Of The University Of Pennsylvania
US Patent
The Trustees Of The University Of Pennsylvania
US Patent
Affinity DataIC50: 1.35E+3nMAssay Description:the inhibitory potency of the individual compounds against the AKR1C isoforms was determined by monitoring the NADP+ dependent oxidation of S-tetralo...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
The Trustees Of The University Of Pennsylvania
US Patent
The Trustees Of The University Of Pennsylvania
US Patent
Affinity DataIC50: 1.50E+3nMAssay Description:the inhibitory potency of the individual compounds against the AKR1C isoforms was determined by monitoring the NADP+ dependent oxidation of S-tetralo...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
The Trustees Of The University Of Pennsylvania
US Patent
The Trustees Of The University Of Pennsylvania
US Patent
Affinity DataIC50: 1.72E+3nMAssay Description:the inhibitory potency of the individual compounds against the AKR1C isoforms was determined by monitoring the NADP+ dependent oxidation of S-tetralo...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
The Trustees Of The University Of Pennsylvania
US Patent
The Trustees Of The University Of Pennsylvania
US Patent
Affinity DataIC50: 1.90E+3nMAssay Description:the inhibitory potency of the individual compounds against the AKR1C isoforms was determined by monitoring the NADP+ dependent oxidation of S-tetralo...More data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 1(Homo sapiens (Human))
The Trustees Of The University Of Pennsylvania
US Patent
The Trustees Of The University Of Pennsylvania
US Patent
Affinity DataIC50: 1.93E+3nMAssay Description:The effect of the compounds on COX-1 activity was determined by a continuous colorimetric assay that monitored the oxidation of N, N, N, N-tetramethy...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
The Trustees Of The University Of Pennsylvania
US Patent
The Trustees Of The University Of Pennsylvania
US Patent
Affinity DataIC50: 2.40E+3nMAssay Description:the inhibitory potency of the individual compounds against the AKR1C isoforms was determined by monitoring the NADP+ dependent oxidation of S-tetralo...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
The Trustees Of The University Of Pennsylvania
US Patent
The Trustees Of The University Of Pennsylvania
US Patent
Affinity DataIC50: 2.75E+3nMAssay Description:the inhibitory potency of the individual compounds against the AKR1C isoforms was determined by monitoring the NADP+ dependent oxidation of S-tetralo...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
The Trustees Of The University Of Pennsylvania
US Patent
The Trustees Of The University Of Pennsylvania
US Patent
Affinity DataIC50: 3.00E+3nMAssay Description:the inhibitory potency of the individual compounds against the AKR1C isoforms was determined by monitoring the NADP+ dependent oxidation of S-tetralo...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
The Trustees Of The University Of Pennsylvania
US Patent
The Trustees Of The University Of Pennsylvania
US Patent
Affinity DataIC50: 4.35E+3nMAssay Description:the inhibitory potency of the individual compounds against the AKR1C isoforms was determined by monitoring the NADP+ dependent oxidation of S-tetralo...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees Of The University Of Pennsylvania
US Patent
The Trustees Of The University Of Pennsylvania
US Patent
Affinity DataIC50: 6.00E+3nMAssay Description:the inhibitory potency of the individual compounds against the AKR1C isoforms was determined by monitoring the NADP+ dependent oxidation of S-tetralo...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
The Trustees Of The University Of Pennsylvania
US Patent
The Trustees Of The University Of Pennsylvania
US Patent
Affinity DataIC50: 6.30E+3nMAssay Description:the inhibitory potency of the individual compounds against the AKR1C isoforms was determined by monitoring the NADP+ dependent oxidation of S-tetralo...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
The Trustees Of The University Of Pennsylvania
US Patent
The Trustees Of The University Of Pennsylvania
US Patent
Affinity DataIC50: 7.60E+3nMAssay Description:the inhibitory potency of the individual compounds against the AKR1C isoforms was determined by monitoring the NADP+ dependent oxidation of S-tetralo...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
The Trustees Of The University Of Pennsylvania
US Patent
The Trustees Of The University Of Pennsylvania
US Patent
Affinity DataIC50: 1.90E+4nMAssay Description:the inhibitory potency of the individual compounds against the AKR1C isoforms was determined by monitoring the NADP+ dependent oxidation of S-tetralo...More data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 2(Homo sapiens (Human))
The Trustees Of The University Of Pennsylvania
US Patent
The Trustees Of The University Of Pennsylvania
US Patent
Affinity DataIC50: >2.50E+4nMAssay Description:The effect of the compounds on COX-1 activity was determined by a continuous colorimetric assay that monitored the oxidation of N, N, N, N-tetramethy...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
The Trustees Of The University Of Pennsylvania
US Patent
The Trustees Of The University Of Pennsylvania
US Patent
Affinity DataIC50: 3.40E+4nMAssay Description:the inhibitory potency of the individual compounds against the AKR1C isoforms was determined by monitoring the NADP+ dependent oxidation of S-tetralo...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
The Trustees Of The University Of Pennsylvania
US Patent
The Trustees Of The University Of Pennsylvania
US Patent
Affinity DataIC50: 4.81E+4nMAssay Description:the inhibitory potency of the individual compounds against the AKR1C isoforms was determined by monitoring the NADP+ dependent oxidation of S-tetralo...More data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 1(Homo sapiens (Human))
The Trustees Of The University Of Pennsylvania
US Patent
The Trustees Of The University Of Pennsylvania
US Patent
Affinity DataIC50: >1.00E+5nMAssay Description:The effect of the compounds on COX-1 activity was determined by a continuous colorimetric assay that monitored the oxidation of N, N, N, N-tetramethy...More data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 1(Homo sapiens (Human))
The Trustees Of The University Of Pennsylvania
US Patent
The Trustees Of The University Of Pennsylvania
US Patent
Affinity DataIC50: >1.00E+5nMAssay Description:The effect of the compounds on COX-1 activity was determined by a continuous colorimetric assay that monitored the oxidation of N, N, N, N-tetramethy...More data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)