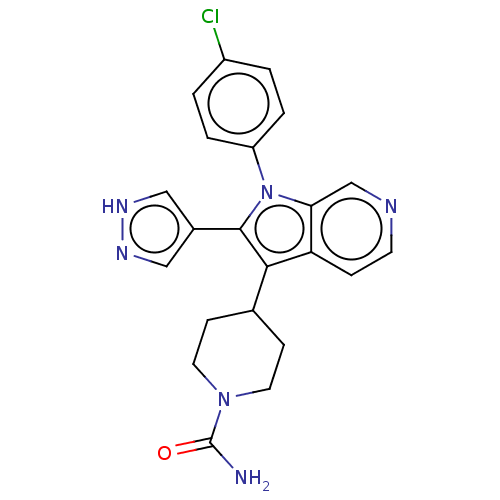
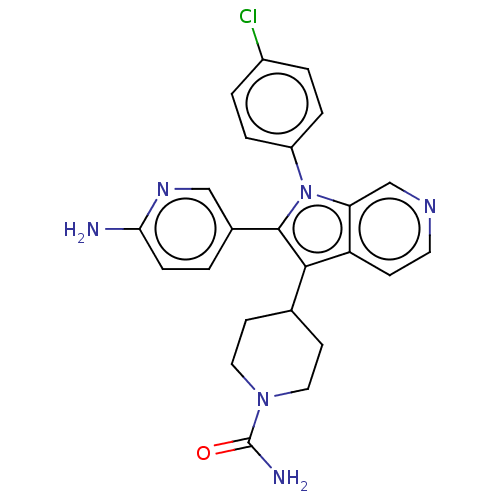
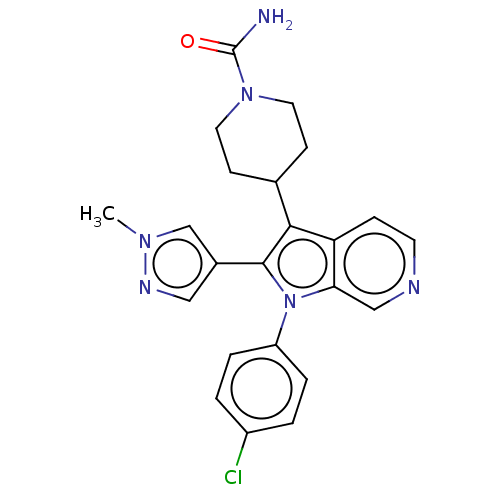
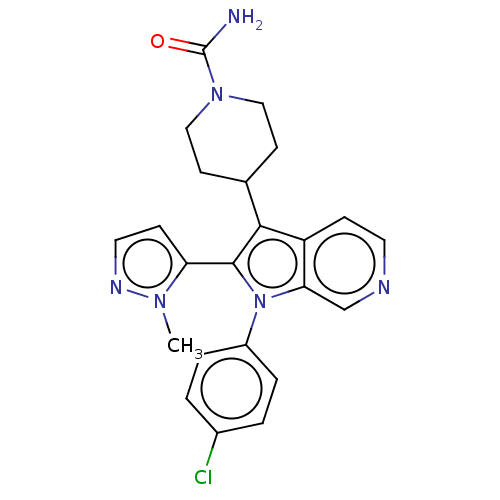
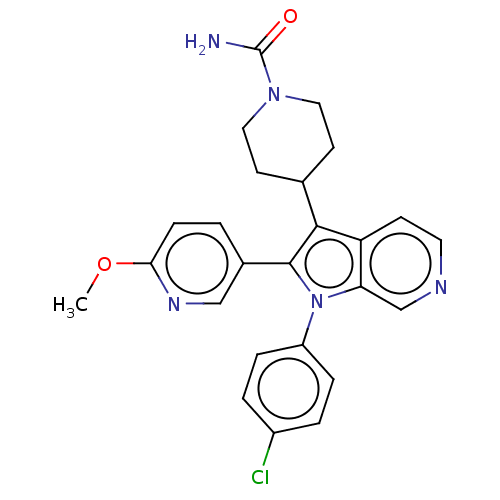
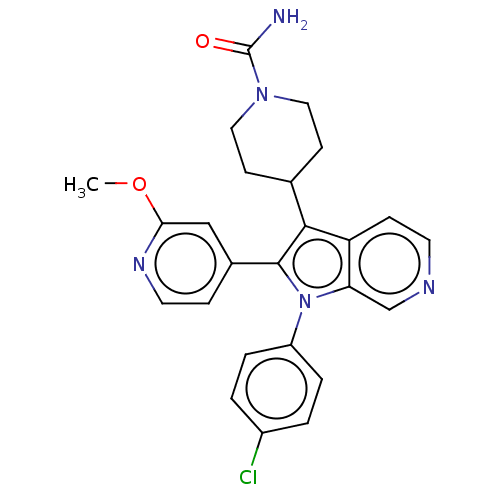
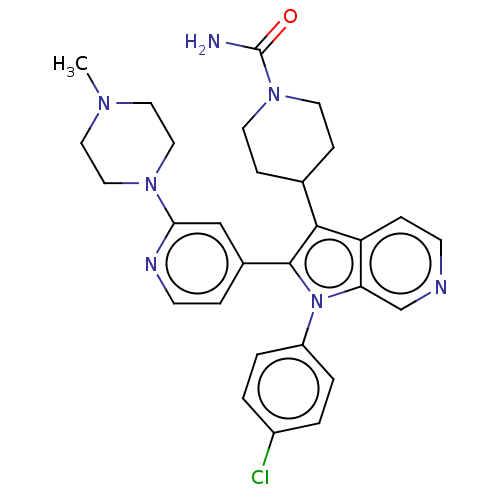
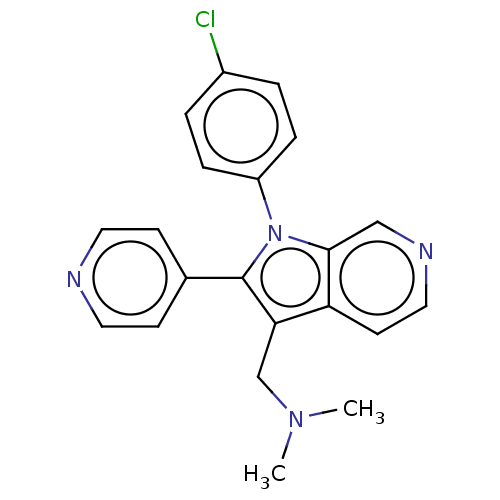
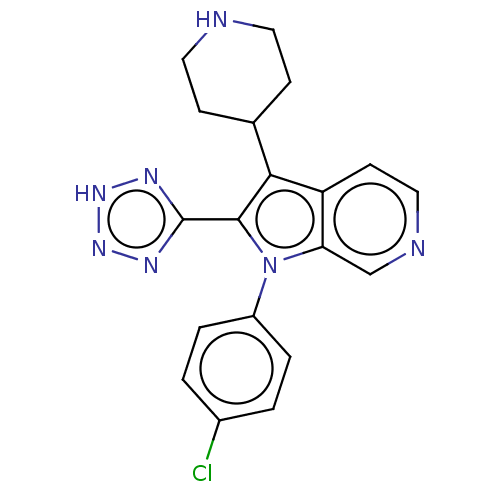
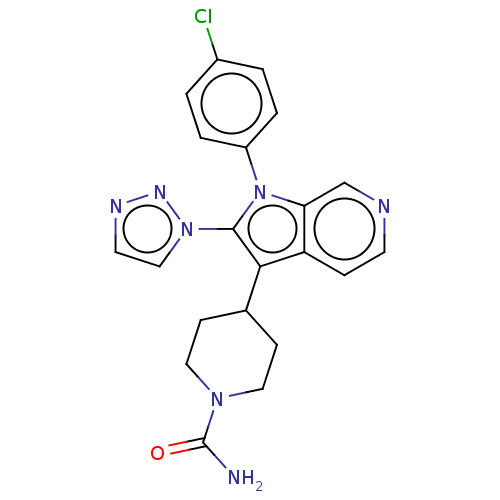
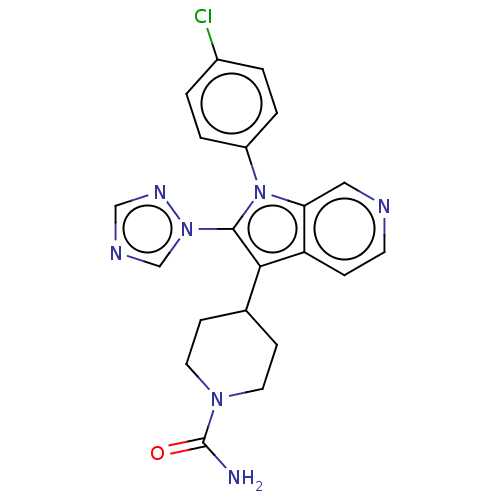
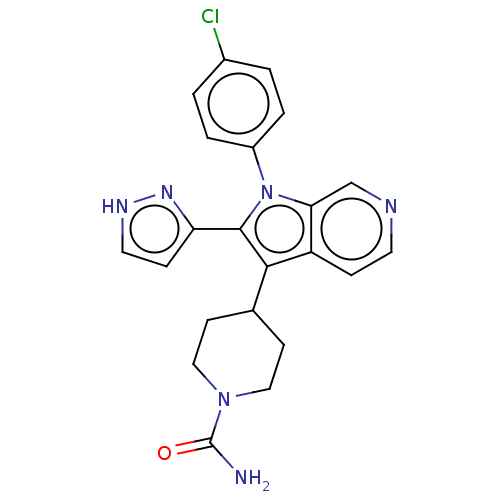
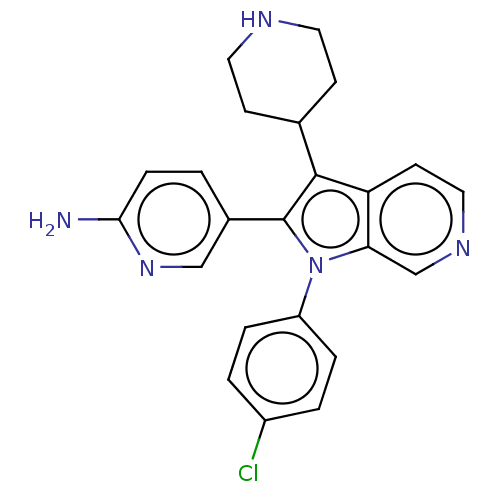
Report error Found 36 Enz. Inhib. hit(s) with all data for entry = 800
Affinity DataIC50: 100nMAssay Description:All primary assays were performed at RT. with purified recombinantly expressed human SSAO. Enzyme was prepared essentially as described in hman et al...More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:All primary assays were performed at RT. with purified recombinantly expressed human SSAO. Enzyme was prepared essentially as described in hman et al...More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:All primary assays were performed at RT. with purified recombinantly expressed human SSAO. Enzyme was prepared essentially as described in hman et al...More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:All primary assays were performed at RT. with purified recombinantly expressed human SSAO. Enzyme was prepared essentially as described in hman et al...More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:All primary assays were performed at RT. with purified recombinantly expressed human SSAO. Enzyme was prepared essentially as described in hman et al...More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:All primary assays were performed at RT. with purified recombinantly expressed human SSAO. Enzyme was prepared essentially as described in hman et al...More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:All primary assays were performed at RT. with purified recombinantly expressed human SSAO. Enzyme was prepared essentially as described in hman et al...More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:All primary assays were performed at RT. with purified recombinantly expressed human SSAO. Enzyme was prepared essentially as described in hman et al...More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:All primary assays were performed at RT. with purified recombinantly expressed human SSAO. Enzyme was prepared essentially as described in hman et al...More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:All primary assays were performed at RT. with purified recombinantly expressed human SSAO. Enzyme was prepared essentially as described in hman et al...More data for this Ligand-Target Pair
Affinity DataIC50: 550nMpH: 7.3 T: 2°CAssay Description:Compounds of the invention were tested for inhibition of the human ether a go-go related gene (hERG) K+ channel using IonWorks patch clamp electrophy...More data for this Ligand-Target Pair
Affinity DataIC50: 550nMpH: 7.3 T: 2°CAssay Description:Compounds of the invention were tested for inhibition of the human ether a go-go related gene (hERG) K+ channel using IonWorks patch clamp electrophy...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:All primary assays were performed at RT. with purified recombinantly expressed human SSAO. Enzyme was prepared essentially as described in hman et al...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:All primary assays were performed at RT. with purified recombinantly expressed human SSAO. Enzyme was prepared essentially as described in hman et al...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:All primary assays were performed at RT. with purified recombinantly expressed human SSAO. Enzyme was prepared essentially as described in hman et al...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:All primary assays were performed at RT. with purified recombinantly expressed human SSAO. Enzyme was prepared essentially as described in hman et al...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:All primary assays were performed at RT. with purified recombinantly expressed human SSAO. Enzyme was prepared essentially as described in hman et al...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:All primary assays were performed at RT. with purified recombinantly expressed human SSAO. Enzyme was prepared essentially as described in hman et al...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:All primary assays were performed at RT. with purified recombinantly expressed human SSAO. Enzyme was prepared essentially as described in hman et al...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:All primary assays were performed at RT. with purified recombinantly expressed human SSAO. Enzyme was prepared essentially as described in hman et al...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:All primary assays were performed at RT. with purified recombinantly expressed human SSAO. Enzyme was prepared essentially as described in hman et al...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:All primary assays were performed at RT. with purified recombinantly expressed human SSAO. Enzyme was prepared essentially as described in hman et al...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:All primary assays were performed at RT. with purified recombinantly expressed human SSAO. Enzyme was prepared essentially as described in hman et al...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMpH: 7.3 T: 2°CAssay Description:Compounds of the invention were tested for inhibition of the human ether a go-go related gene (hERG) K+ channel using IonWorks patch clamp electrophy...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMpH: 7.3 T: 2°CAssay Description:Compounds of the invention were tested for inhibition of the human ether a go-go related gene (hERG) K+ channel using IonWorks patch clamp electrophy...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMpH: 7.3 T: 2°CAssay Description:Compounds of the invention were tested for inhibition of the human ether a go-go related gene (hERG) K+ channel using IonWorks patch clamp electrophy...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMpH: 7.3 T: 2°CAssay Description:Compounds of the invention were tested for inhibition of the human ether a go-go related gene (hERG) K+ channel using IonWorks patch clamp electrophy...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMpH: 7.3 T: 2°CAssay Description:Compounds of the invention were tested for inhibition of the human ether a go-go related gene (hERG) K+ channel using IonWorks patch clamp electrophy...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMpH: 7.3 T: 2°CAssay Description:Compounds of the invention were tested for inhibition of the human ether a go-go related gene (hERG) K+ channel using IonWorks patch clamp electrophy...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMpH: 7.3 T: 2°CAssay Description:Compounds of the invention were tested for inhibition of the human ether a go-go related gene (hERG) K+ channel using IonWorks patch clamp electrophy...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMpH: 7.3 T: 2°CAssay Description:Compounds of the invention were tested for inhibition of the human ether a go-go related gene (hERG) K+ channel using IonWorks patch clamp electrophy...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMpH: 7.3 T: 2°CAssay Description:Compounds of the invention were tested for inhibition of the human ether a go-go related gene (hERG) K+ channel using IonWorks patch clamp electrophy...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMpH: 7.3 T: 2°CAssay Description:Compounds of the invention were tested for inhibition of the human ether a go-go related gene (hERG) K+ channel using IonWorks patch clamp electrophy...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMpH: 7.3 T: 2°CAssay Description:Compounds of the invention were tested for inhibition of the human ether a go-go related gene (hERG) K+ channel using IonWorks patch clamp electrophy...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMpH: 7.3 T: 2°CAssay Description:Compounds of the invention were tested for inhibition of the human ether a go-go related gene (hERG) K+ channel using IonWorks patch clamp electrophy...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMpH: 7.3 T: 2°CAssay Description:Compounds of the invention were tested for inhibition of the human ether a go-go related gene (hERG) K+ channel using IonWorks patch clamp electrophy...More data for this Ligand-Target Pair