Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Cathepsin D
Ligand
BDBM358
Substrate
Peptide Substrate
Meas. Tech.
Enzyme Inhibition Measurements
Ki
>2000±n/a nM
Citation
 Ersmark, K; Feierberg, I; Bjelic, S; Hamelink, E; Hackett, F; Blackman, MJ; Hulten, J; Samuelsson, B; Aqvist, J; Hallberg, A Potent inhibitors of the Plasmodium falciparum enzymes plasmepsin I and II devoid of cathepsin D inhibitory activity. J Med Chem 47:110-22 (2004) [PubMed] Article
Ersmark, K; Feierberg, I; Bjelic, S; Hamelink, E; Hackett, F; Blackman, MJ; Hulten, J; Samuelsson, B; Aqvist, J; Hallberg, A Potent inhibitors of the Plasmodium falciparum enzymes plasmepsin I and II devoid of cathepsin D inhibitory activity. J Med Chem 47:110-22 (2004) [PubMed] Article More Info.:
Target
Name:
Cathepsin D
Synonyms:
CATD_HUMAN | CPSD | CTSD | Cathepsin D [Precursor] | Cathepsin D heavy chain | Cathepsin D light chain | Cathepsin D precursor
Type:
Enzyme
Mol. Mass.:
44551.72
Organism:
Homo sapiens (Human)
Description:
Human proCathepsin D (SwissProt accession number P07339) was expressed in Sf9 cells, purified, and autoactivated.
Residue:
412
Sequence:
MQPSSLLPLALCLLAAPASALVRIPLHKFTSIRRTMSEVGGSVEDLIAKGPVSKYSQAVPAVTEGPIPEVLKNYMDAQYYGEIGIGTPPQCFTVVFDTGSSNLWVPSIHCKLLDIACWIHHKYNSDKSSTYVKNGTSFDIHYGSGSLSGYLSQDTVSVPCQSASSASALGGVKVERQVFGEATKQPGITFIAAKFDGILGMAYPRISVNNVLPVFDNLMQQKLVDQNIFSFYLSRDPDAQPGGELMLGGTDSKYYKGSLSYLNVTRKAYWQVHLDQVEVASGLTLCKEGCEAIVDTGTSLMVGPVDEVRELQKAIGAVPLIQGEYMIPCEKVSTLPAITLKLGGKGYKLSPEDYTLKVSQAGKTLCLSGFMGMDIPPPSGPLWILGDVFIGRYYTVFDRDNNRVGFAEAARL
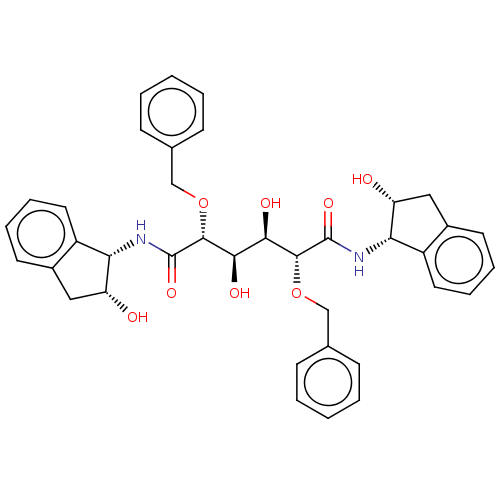
Inhibitor
Name:
BDBM358
Synonyms:
(2R,3R,4R,5R)-2,5-bis(benzyloxy)-3,4-dihydroxy-N,N'-bis[(1S,2R)-2-hydroxy-2,3-dihydro-1H-inden-1-yl]hexanediamide | 1-Valine Methylamide deriv. 11 | C2-symmetric compound 8 | Diol-Based HIV-1 protease inhibitor 1 | symmetric/asymmetric inhibitor 2
Type:
Small organic molecule
Emp. Form.:
C38H40N2O8
Mol. Mass.:
652.7328
SMILES:
O[C@H]([C@@H](O)[C@@H](OCc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12)[C@@H](OCc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12
Substrate
Name:
Peptide Substrate
Synonyms:
n/a
Type:
Peptide
Mol. Mass.:
3501.67
Organism:
n/a
Description:
n/a
Residue:
31
Sequence:
DACYLGLARGNLEPHELESERPHEPREDANS