Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Histone-lysine N-methyltransferase EZH2
Ligand
BDBM636210
Substrate
n/a
Meas. Tech.
EZH2 Enzyme Activity Assay
IC50
0.460±n/a nM
Citation
More Info.:
Target
Name:
Histone-lysine N-methyltransferase EZH2
Synonyms:
ENX-1 | EZH2 | EZH2_HUMAN | Enhancer of zeste homolog 2 (EZH2) | Histone-lysine N-methyltransferase EZH2 | KMT6 | Lysine N-methyltransferase 6
Type:
Protein
Mol. Mass.:
85367.84
Organism:
Homo sapiens (Human)
Description:
Q15910
Residue:
746
Sequence:
MGQTGKKSEKGPVCWRKRVKSEYMRLRQLKRFRRADEVKSMFSSNRQKILERTEILNQEWKQRRIQPVHILTSVSSLRGTRECSVTSDLDFPTQVIPLKTLNAVASVPIMYSWSPLQQNFMVEDETVLHNIPYMGDEVLDQDGTFIEELIKNYDGKVHGDRECGFINDEIFVELVNALGQYNDDDDDDDGDDPEEREEKQKDLEDHRDDKESRPPRKFPSDKIFEAISSMFPDKGTAEELKEKYKELTEQQLPGALPPECTPNIDGPNAKSVQREQSLHSFHTLFCRRCFKYDCFLHPFHATPNTYKRKNTETALDNKPCGPQCYQHLEGAKEFAAALTAERIKTPPKRPGGRRRGRLPNNSSRPSTPTINVLESKDTDSDREAGTETGGENNDKEEEEKKDETSSSSEANSRCQTPIKMKPNIEPPENVEWSGAEASMFRVLIGTYYDNFCAIARLIGTKTCRQVYEFRVKESSIIAPAPAEDVDTPPRKKKRKHRLWAAHCRKIQLKKDGSSNHVYNYQPCDHPRQPCDSSCPCVIAQNFCEKFCQCSSECQNRFPGCRCKAQCNTKQCPCYLAVRECDPDLCLTCGAADHWDSKNVSCKNCSIQRGSKKHLLLAPSDVAGWGIFIKDPVQKNEFISEYCGEIISQDEADRRGKVYDKYMCSFLFNLNNDFVVDATRKGNKIRFANHSVNPNCYAKVMMVNGDHRIGIFAKRAIQTGEELFFDYRYSQADALKYVGIEREMEIP
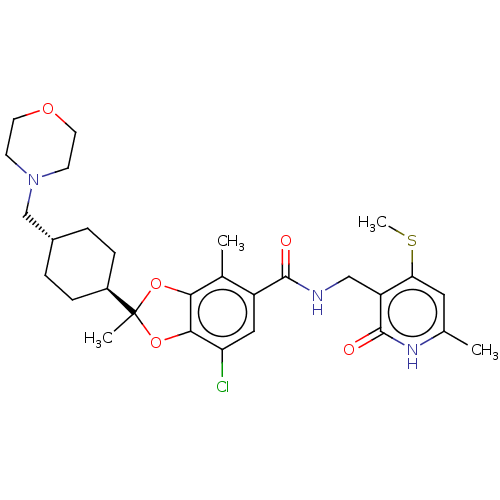
Inhibitor
Name:
BDBM636210
Synonyms:
US20230365541, Compound 57, isomer 1
Type:
Small organic molecule
Emp. Form.:
C29H38ClN3O5S
Mol. Mass.:
576.147
SMILES:
CSc1cc(C)[nH]c(=O)c1CNC(=O)c1cc(Cl)c2OC(C)(Oc2c1C)[C@H]1CC[C@H](CN2CCOCC2)CC1 |r,wU:29.32,wD:26.28,(-5.9,-26.5,;-5.9,-24.96,;-7.23,-24.19,;-8.57,-24.96,;-9.9,-24.19,;-11.24,-24.96,;-9.9,-22.65,;-8.57,-21.88,;-8.57,-20.34,;-7.23,-22.65,;-5.9,-21.88,;-4.57,-22.65,;-3.23,-21.88,;-3.23,-20.34,;-1.9,-22.65,;-1.9,-24.19,;-.57,-24.96,;-.57,-26.5,;.77,-24.19,;2.23,-24.66,;3.14,-23.42,;4.47,-24.19,;2.23,-22.17,;.77,-22.65,;-.57,-21.88,;-.57,-20.34,;4.47,-22.65,;5.8,-23.42,;7.14,-22.65,;7.14,-21.11,;8.47,-20.34,;9.81,-21.11,;9.81,-22.65,;11.14,-23.42,;12.47,-22.65,;12.47,-21.11,;11.14,-20.34,;5.8,-20.34,;4.47,-21.11,)|