Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Oxysterols receptor LXR-beta
Ligand
BDBM28138
Substrate
BDBM19993
Meas. Tech.
Radioligand Binding Assay and Luciferase Transcriptional Reporter Gene Assay
IC50
21±n/a nM
EC50
430±n/a nM
Citation
 Ratni, H; Blum-Kaelin, D; Dehmlow, H; Hartman, P; Jablonski, P; Masciadri, R; Maugeais, C; Patiny-Adam, A; Panday, N; Wright, M Discovery of tetrahydro-cyclopenta[b]indole as selective LXRs modulator. Bioorg Med Chem Lett 19:1654-7 (2009) [PubMed] Article
Ratni, H; Blum-Kaelin, D; Dehmlow, H; Hartman, P; Jablonski, P; Masciadri, R; Maugeais, C; Patiny-Adam, A; Panday, N; Wright, M Discovery of tetrahydro-cyclopenta[b]indole as selective LXRs modulator. Bioorg Med Chem Lett 19:1654-7 (2009) [PubMed] Article More Info.:
Target
Name:
Oxysterols receptor LXR-beta
Synonyms:
LXRB | Liver X receptor beta (NR1H2) | Liver X, LXR beta | NER | NR1H2 | NR1H2_HUMAN | Nuclear receptor NER | UNR | Ubiquitously-expressed nuclear receptor
Type:
Enzyme Catalytic Domain
Mol. Mass.:
50978.79
Organism:
Homo sapiens (Human)
Description:
P55055
Residue:
460
Sequence:
MSSPTTSSLDTPLPGNGPPQPGAPSSSPTVKEEGPEPWPGGPDPDVPGTDEASSACSTDWVIPDPEEEPERKRKKGPAPKMLGHELCRVCGDKASGFHYNVLSCEGCKGFFRRSVVRGGARRYACRGGGTCQMDAFMRRKCQQCRLRKCKEAGMREQCVLSEEQIRKKKIRKQQQESQSQSQSPVGPQGSSSSASGPGASPGGSEAGSQGSGEGEGVQLTAAQELMIQQLVAAQLQCNKRSFSDQPKVTPWPLGADPQSRDARQQRFAHFTELAIISVQEIVDFAKQVPGFLQLGREDQIALLKASTIEIMLLETARRYNHETECITFLKDFTYSKDDFHRAGLQVEFINPIFEFSRAMRRLGLDDAEYALLIAINIFSADRPNVQEPGRVEALQQPYVEALLSYTRIKRPQDQLRFPRMLMKLVSLRTLSSVHSEQVFALRLQDKKLPPLLSEIWDVHE
Inhibitor
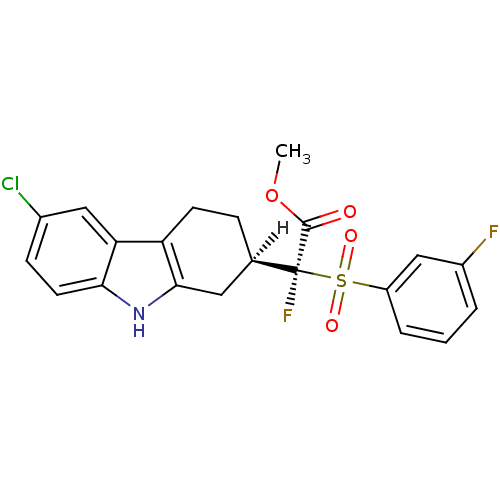
Name:
BDBM28138
Synonyms:
methyl (2S)-2-[(2R)-6-chloro-2,3,4,9-tetrahydro-1H-carbazol-2-yl]-2-fluoro-2-[(3-fluorobenzene)sulfonyl]acetate | tetrahydro-carbazole, (+/-)18
Type:
Small organic molecule
Emp. Form.:
C21H18ClF2NO4S
Mol. Mass.:
453.887
SMILES:
[H][C@]1(CCc2c(C1)[nH]c1ccc(Cl)cc21)[C@@](F)(C(=O)OC)S(=O)(=O)c1cccc(F)c1 |r|
Substrate
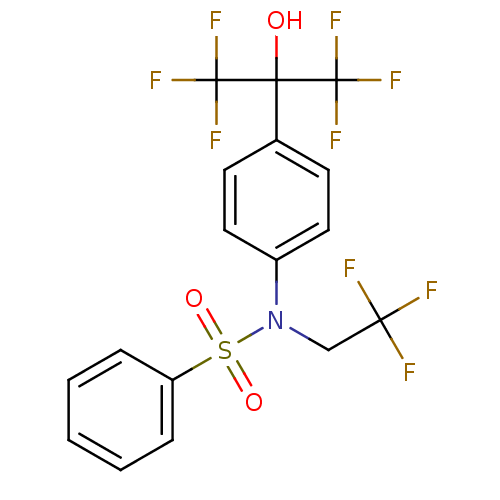
Name:
BDBM19993
Synonyms:
CHEMBL62136 | N-[4-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)phenyl]-N-(2,2,2-trifluoroethyl)benzenesulfonamide | T 0901317 | T0901317 | TO-901317 | US10543183, Compound TO901317 | US10669296, Compound TO901317 | US10945978, Compound 1 | [3H]T0901317
Type:
Small organic molecule
Emp. Form.:
C17H12F9NO3S
Mol. Mass.:
481.333
SMILES:
OC(c1ccc(cc1)N(CC(F)(F)F)S(=O)(=O)c1ccccc1)(C(F)(F)F)C(F)(F)F