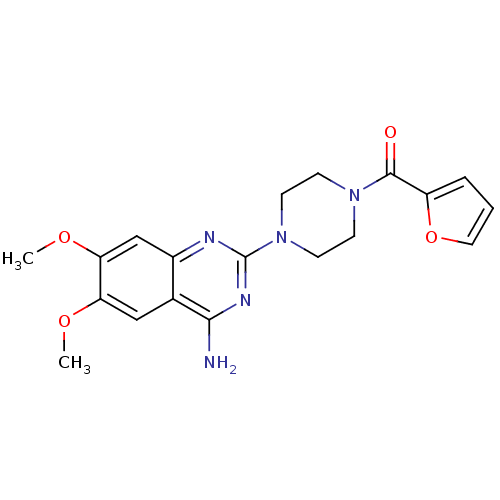
Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
5-hydroxytryptamine receptor 1A
Ligand
BDBM29568
Substrate
n/a
Meas. Tech.
ChEMBL_1508 (CHEMBL616340)
Ki
2344±n/a nM
Citation
 Giardin�, D; Crucianelli, M; Romanelli, R; Leonardi, A; Poggesi, E; Melchiorre, C Synthesis and biological profile of the enantiomers of [4-(4-amino-6,7-dimethoxyquinazolin-2-yl)-cis-octahydroquinoxalin- 1-yl]furan-2-ylmethanone (cyclazosin), a potent competitive alpha 1B- adrenoceptor antagonist. J Med Chem 39:4602-7 (1996) [PubMed] Article
Giardin�, D; Crucianelli, M; Romanelli, R; Leonardi, A; Poggesi, E; Melchiorre, C Synthesis and biological profile of the enantiomers of [4-(4-amino-6,7-dimethoxyquinazolin-2-yl)-cis-octahydroquinoxalin- 1-yl]furan-2-ylmethanone (cyclazosin), a potent competitive alpha 1B- adrenoceptor antagonist. J Med Chem 39:4602-7 (1996) [PubMed] Article More Info.:
Target
Name:
5-hydroxytryptamine receptor 1A
Synonyms:
5-HT-1A | 5-HT1 | 5-HT1A | 5-Hydroxytryptamine receptor 1A (5-HT1A) | 5-hydroxytryptamine receptor 1A (5HT1A) | 5HT1A_RAT | 5ht1a | G-21 | Htr1a | Serotonin 1 (5-HT1) receptor | Serotonin 1a (5-HT1a) receptor/Adrenergic receptor alpha-1 | Serotonin receptor 1A
Type:
G Protein-Coupled Receptor (GPCR)
Mol. Mass.:
46445.29
Organism:
Rattus norvegicus (rat)
Description:
Binding assays were performed using rat hippocampal membranes.
Residue:
422
Sequence:
MDVFSFGQGNNTTASQEPFGTGGNVTSISDVTFSYQVITSLLLGTLIFCAVLGNACVVAAIALERSLQNVANYLIGSLAVTDLMVSVLVLPMAALYQVLNKWTLGQVTCDLFIALDVLCCTSSILHLCAIALDRYWAITDPIDYVNKRTPRRAAALISLTWLIGFLISIPPMLGWRTPEDRSDPDACTISKDHGYTIYSTFGAFYIPLLLMLVLYGRIFRAARFRIRKTVRKVEKKGAGTSLGTSSAPPPKKSLNGQPGSGDWRRCAENRAVGTPCTNGAVRQGDDEATLEVIEVHRVGNSKEHLPLPSESGSNSYAPACLERKNERNAEAKRKMALARERKTVKTLGIIMGTFILCWLPFFIVALVLPFCESSCHMPALLGAIINWLGYSNSLLNPVIYAYFNKDFQNAFKKIIKCKFCRR