Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Adenosine receptor A2b
Ligand
BDBM50004566
Substrate
n/a
Meas. Tech.
ChEMBL_30295 (CHEMBL875505)
IC50
1200±n/a nM
Citation
 Kim, YC; de Zwart, M; Chang, L; Moro, S; von Frijtag Drabbe Künzel, JK; Melman, N; IJzerman, AP; Jacobson, KA Derivatives of the triazoloquinazoline adenosine antagonist (CGS 15943) having high potency at the human A2B and A3 receptor subtypes. J Med Chem 41:2835-45 (1998) [PubMed] Article
Kim, YC; de Zwart, M; Chang, L; Moro, S; von Frijtag Drabbe Künzel, JK; Melman, N; IJzerman, AP; Jacobson, KA Derivatives of the triazoloquinazoline adenosine antagonist (CGS 15943) having high potency at the human A2B and A3 receptor subtypes. J Med Chem 41:2835-45 (1998) [PubMed] Article More Info.:
Target
Name:
Adenosine receptor A2b
Synonyms:
AA2BR_HUMAN | ADENOSINE A2B | ADORA2B | Adenosine receptor A2B (A2B) | Adenosine receptors A2b | Adenosine receptors; A2a & A2b
Type:
G Protein-Coupled Receptor (GPCR)
Mol. Mass.:
36341.22
Organism:
Homo sapiens (Human)
Description:
n/a
Residue:
332
Sequence:
MLLETQDALYVALELVIAALSVAGNVLVCAAVGTANTLQTPTNYFLVSLAAADVAVGLFAIPFAITISLGFCTDFYGCLFLACFVLVLTQSSIFSLLAVAVDRYLAICVPLRYKSLVTGTRARGVIAVLWVLAFGIGLTPFLGWNSKDSATNNCTEPWDGTTNESCCLVKCLFENVVPMSYMVYFNFFGCVLPPLLIMLVIYIKIFLVACRQLQRTELMDHSRTTLQREIHAAKSLAMIVGIFALCWLPVHAVNCVTLFQPAQGKNKPKWAMNMAILLSHANSVVNPIVYAYRNRDFRYTFHKIISRYLLCQADVKSGNGQAGVQPALGVGL
Inhibitor
Name:
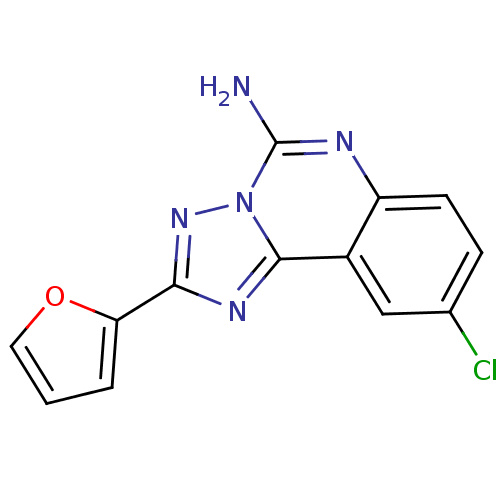
BDBM50004566
Synonyms:
9-Chloro-2-furan-2-yl-[1,2,4]triazolo[1,5-c]quinazolin-5-ylamine | 9-Chloro-2-furan-2-yl-[1,2,4]triazolo[1,5-c]quinazolin-5-ylamine(CGS 15943) | 9-Chloro-[1,2,4]triazolo[1,5-c]quinazolin-5-ylamine(CGS 15943) | 9-chloro-2-(2-furanyl)-1,2,4-triazolo[1.5-c]quinazolin-5-amine | 9-chloro-2-(furan-2-yl)-[1,2,4]triazolo[1,5-c]quinazolin-5-amine | CGS-15943 | CHEMBL16687 | CHEMBL268431 | Nonnucleoside analog, 4
Type:
Small organic molecule
Emp. Form.:
C13H8ClN5O
Mol. Mass.:
285.689
SMILES:
Nc1nc2ccc(Cl)cc2c2nc(nn12)-c1ccco1