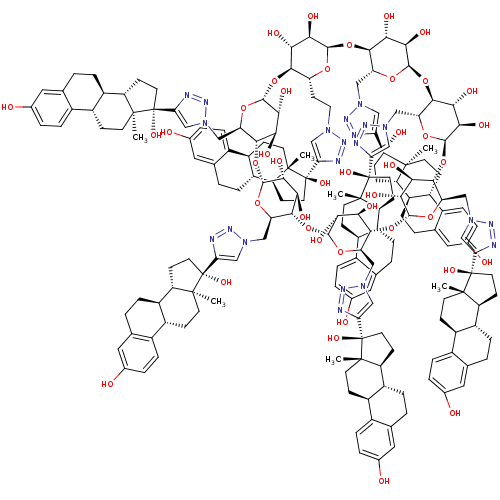
Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Estrogen receptor
Ligand
BDBM50309553
Substrate
n/a
Meas. Tech.
ChEMBL_610114 (CHEMBL1074479)
EC50
200±n/a nM
Citation
 Kim, HY; Sohn, J; Wijewickrama, GT; Edirisinghe, P; Gherezghiher, T; Hemachandra, M; Lu, PY; Chandrasena, RE; Molloy, ME; Tonetti, DA; Thatcher, GR Click synthesis of estradiol-cyclodextrin conjugates as cell compartment selective estrogens. Bioorg Med Chem 18:809-21 (2010) [PubMed] Article
Kim, HY; Sohn, J; Wijewickrama, GT; Edirisinghe, P; Gherezghiher, T; Hemachandra, M; Lu, PY; Chandrasena, RE; Molloy, ME; Tonetti, DA; Thatcher, GR Click synthesis of estradiol-cyclodextrin conjugates as cell compartment selective estrogens. Bioorg Med Chem 18:809-21 (2010) [PubMed] Article More Info.:
Target
Name:
Estrogen receptor
Synonyms:
ER | ER-alpha | ESR | ESR1 | ESR1_HUMAN | Estradiol receptor | Estrogen receptor | Estrogen receptor (ER alpha) | Estrogen receptor (ER-alpha) | Estrogen receptor alpha (ER alpha) | Estrogen receptor alpha (ER) | NR3A1 | Nuclear receptor subfamily 3 group A member 1
Type:
Protein
Mol. Mass.:
66230.44
Organism:
Homo sapiens (Human)
Description:
P03372
Residue:
595
Sequence:
MTMTLHTKASGMALLHQIQGNELEPLNRPQLKIPLERPLGEVYLDSSKPAVYNYPEGAAYEFNAAAAANAQVYGQTGLPYGPGSEAAAFGSNGLGGFPPLNSVSPSPLMLLHPPPQLSPFLQPHGQQVPYYLENEPSGYTVREAGPPAFYRPNSDNRRQGGRERLASTNDKGSMAMESAKETRYCAVCNDYASGYHYGVWSCEGCKAFFKRSIQGHNDYMCPATNQCTIDKNRRKSCQACRLRKCYEVGMMKGGIRKDRRGGRMLKHKRQRDDGEGRGEVGSAGDMRAANLWPSPLMIKRSKKNSLALSLTADQMVSALLDAEPPILYSEYDPTRPFSEASMMGLLTNLADRELVHMINWAKRVPGFVDLTLHDQVHLLECAWLEILMIGLVWRSMEHPGKLLFAPNLLLDRNQGKCVEGMVEIFDMLLATSSRFRMMNLQGEEFVCLKSIILLNSGVYTFLSSTLKSLEEKDHIHRVLDKITDTLIHLMAKAGLTLQQQHQRLAQLLLILSHIRHMSNKGMEHLYSMKCKNVVPLYDLLLEMLDAHRLHAPTSRGGASVEETDQSHLATAGSTSSHSLQKYYITGEAEGFPATV
Inhibitor
Name:
BDBM50309553
Synonyms:
CHEMBL605828 | Per-6-(estradiol-17-(1H-1,2,3-triazol-4-yl))-6-deoxy-beta cyclodextrin
Type:
Small organic molecule
Emp. Form.:
C183H233N21O42
Mol. Mass.:
3398.9236
SMILES:
C[C@]12CC[C@H]3[C@@H](CCc4cc(O)ccc34)[C@@H]1CC[C@@]2(O)c1cn(CC[C@H]2O[C@@H]3O[C@@H]4[C@@H](Cn5cc(nn5)[C@]5(O)CC[C@H]6[C@@H]7CCc8cc(O)ccc8[C@H]7CC[C@]56C)O[C@H](O[C@@H]5[C@@H](Cn6cc(nn6)[C@]6(O)CC[C@H]7[C@@H]8CCc9cc(O)ccc9[C@H]8CC[C@]67C)O[C@H](O[C@@H]6[C@@H](Cn7cc(nn7)[C@]7(O)CC[C@H]8[C@@H]9CCc%10cc(O)ccc%10[C@H]9CC[C@]78C)O[C@H](O[C@@H]7[C@@H](Cn8cc(nn8)[C@]8(O)CC[C@H]9[C@@H]%10CCc%11cc(O)ccc%11[C@H]%10CC[C@]89C)O[C@H](O[C@@H]8[C@@H](Cn9cc(nn9)[C@]9(O)CC[C@H]%10[C@@H]%11CCc%12cc(O)ccc%12[C@H]%11CC[C@]9%10C)O[C@H](O[C@@H]9[C@@H](Cn%10cc(nn%10)[C@]%10(O)CC[C@H]%11[C@@H]%12CCc%13cc(O)ccc%13[C@H]%12CC[C@]%10%11C)O[C@H](O[C@H]2[C@H](O)[C@H]3O)[C@H](O)[C@H]9O)[C@H](O)[C@H]8O)[C@H](O)[C@H]7O)[C@H](O)[C@H]6O)[C@H](O)[C@H]5O)[C@H](O)[C@H]4O)nn1 |r|