Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Atypical kinase COQ8A, mitochondrial
Ligand
BDBM50314070
Substrate
n/a
Meas. Tech.
ChEMBL_742471 (CHEMBL1769119)
Kd
>1000±n/a nM
Citation
 Goldstein, DM; Soth, M; Gabriel, T; Dewdney, N; Kuglstatter, A; Arzeno, H; Chen, J; Bingenheimer, W; Dalrymple, SA; Dunn, J; Farrell, R; Frauchiger, S; La Fargue, J; Ghate, M; Graves, B; Hill, RJ; Li, F; Litman, R; Loe, B; McIntosh, J; McWeeney, D; Papp, E; Park, J; Reese, HF; Roberts, RT; Rotstein, D; San Pablo, B; Sarma, K; Stahl, M; Sung, ML; Suttman, RT; Sjogren, EB; Tan, Y; Trejo, A; Welch, M; Weller, P; Wong, BR; Zecic, H Discovery of 6-(2,4-difluorophenoxy)-2-[3-hydroxy-1-(2-hydroxyethyl)propylamino]-8-methyl-8H-pyrido[2,3-d]pyrimidin-7-one (pamapimod) and 6-(2,4-difluorophenoxy)-8-methyl-2-(tetrahydro-2H-pyran-4-ylamino)pyrido[2,3-d]pyrimidin-7(8H)-one (R1487) as orally bioavailable and highly selective inhibitors J Med Chem 54:2255-65 (2011) [PubMed] Article
Goldstein, DM; Soth, M; Gabriel, T; Dewdney, N; Kuglstatter, A; Arzeno, H; Chen, J; Bingenheimer, W; Dalrymple, SA; Dunn, J; Farrell, R; Frauchiger, S; La Fargue, J; Ghate, M; Graves, B; Hill, RJ; Li, F; Litman, R; Loe, B; McIntosh, J; McWeeney, D; Papp, E; Park, J; Reese, HF; Roberts, RT; Rotstein, D; San Pablo, B; Sarma, K; Stahl, M; Sung, ML; Suttman, RT; Sjogren, EB; Tan, Y; Trejo, A; Welch, M; Weller, P; Wong, BR; Zecic, H Discovery of 6-(2,4-difluorophenoxy)-2-[3-hydroxy-1-(2-hydroxyethyl)propylamino]-8-methyl-8H-pyrido[2,3-d]pyrimidin-7-one (pamapimod) and 6-(2,4-difluorophenoxy)-8-methyl-2-(tetrahydro-2H-pyran-4-ylamino)pyrido[2,3-d]pyrimidin-7(8H)-one (R1487) as orally bioavailable and highly selective inhibitors J Med Chem 54:2255-65 (2011) [PubMed] Article More Info.:
Target
Name:
Atypical kinase COQ8A, mitochondrial
Synonyms:
ADCK3 | CABC1 | COQ8A | COQ8A_HUMAN | Chaperone activity of bc1 complex-like, mitochondrial
Type:
PROTEIN
Mol. Mass.:
71954.89
Organism:
Homo sapiens (Human)
Description:
ChEMBL_655673
Residue:
647
Sequence:
MAAILGDTIMVAKGLVKLTQAAVETHLQHLGIGGELIMAARALQSTAVEQIGMFLGKVQGQDKHEEYFAENFGGPEGEFHFSVPHAAGASTDFSSASAPDQSAPPSLGHAHSEGPAPAYVASGPFREAGFPGQASSPLGRANGRLFANPRDSFSAMGFQRRFFHQDQSPVGGLTAEDIEKARQAKARPENKQHKQTLSEHARERKVPVTRIGRLANFGGLAVGLGFGALAEVAKKSLRSEDPSGKKAVLGSSPFLSEANAERIVRTLCKVRGAALKLGQMLSIQDDAFINPHLAKIFERVRQSADFMPLKQMMKTLNNDLGPNWRDKLEYFEERPFAAASIGQVHLARMKGGREVAMKIQYPGVAQSINSDVNNLMAVLNMSNMLPEGLFPEHLIDVLRRELALECDYQREAACARKFRDLLKGHPFFYVPEIVDELCSPHVLTTELVSGFPLDQAEGLSQEIRNEICYNILVLCLRELFEFHFMQTDPNWSNFFYDPQQHKVALLDFGATREYDRSFTDLYIQIIRAAADRDRETVRAKSIEMKFLTGYEVKVMEDAHLDAILILGEAFASDEPFDFGTQSTTEKIHNLIPVMLRHRLVPPPEETYSLHRKMGGSFLICSKLKARFPCKAMFEEAYSNYCKRQAQQ
Inhibitor
Name:
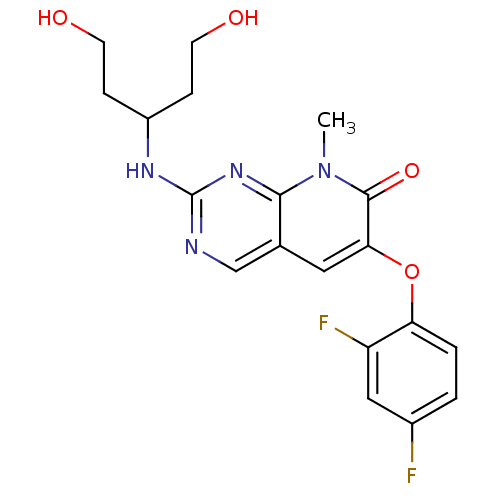
BDBM50314070
Synonyms:
6-(2,4-difluorophenoxy)-2-{[3-hydroxy-1-(2-hydroxyethyl)propyl]amino}-8-methylpyrido[2,3-d]pyrimidin-7(8H)-one | CHEMBL1090089 | pamapimod
Type:
Small organic molecule
Emp. Form.:
C19H20F2N4O4
Mol. Mass.:
406.3833
SMILES:
Cn1c2nc(NC(CCO)CCO)ncc2cc(Oc2ccc(F)cc2F)c1=O