Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Prostaglandin G/H synthase 2
Ligand
BDBM205485
Substrate
n/a
Meas. Tech.
COX Enzyme Inhibition Assay (Table 1)
IC50
>2.5e+4±n/a nM
Citation
 Uddin, MJ; Crews, BC; Xu, S; Ghebreselasie, K; Daniel, CK; Kingsley, PJ; Banerjee, S; Marnett, LJ Antitumor Activity of Cytotoxic Cyclooxygenase-2 Inhibitors. ACS Chem Biol 11:3052-3060 (2016) [PubMed] Article
Uddin, MJ; Crews, BC; Xu, S; Ghebreselasie, K; Daniel, CK; Kingsley, PJ; Banerjee, S; Marnett, LJ Antitumor Activity of Cytotoxic Cyclooxygenase-2 Inhibitors. ACS Chem Biol 11:3052-3060 (2016) [PubMed] Article More Info.:
Target
Name:
Prostaglandin G/H synthase 2
Synonyms:
Cox-2 | Cox2 | Cyclooxygenase-2 | Cyclooxygenase-2 (COX-2) | Glucocorticoid-regulated inflammatory cyclooxygenase | Gripghs | Macrophage activation-associated marker protein P71/73 | PES-2 | PGH synthase 2 | PGH2_MOUSE | PGHS-2 | PHS II | Pghs-b | Prostaglandin G/H synthase (cyclooxygenase) | Prostaglandin H2 synthase 2 | Prostaglandin-endoperoxide synthase 2 | Ptgs2 | TIS10 protein | Tis10
Type:
Protein
Mol. Mass.:
69020.39
Organism:
Mus musculus (Mouse)
Description:
Q05769
Residue:
604
Sequence:
MLFRAVLLCAALGLSQAANPCCSNPCQNRGECMSTGFDQYKCDCTRTGFYGENCTTPEFLTRIKLLLKPTPNTVHYILTHFKGVWNIVNNIPFLRSLIMKYVLTSRSYLIDSPPTYNVHYGYKSWEAFSNLSYYTRALPPVADDCPTPMGVKGNKELPDSKEVLEKVLLRREFIPDPQGSNMMFAFFAQHFTHQFFKTDHKRGPGFTRGLGHGVDLNHIYGETLDRQHKLRLFKDGKLKYQVIGGEVYPPTVKDTQVEMIYPPHIPENLQFAVGQEVFGLVPGLMMYATIWLREHNRVCDILKQEHPEWGDEQLFQTSRLILIGETIKIVIEDYVQHLSGYHFKLKFDPELLFNQQFQYQNRIASEFNTLYHWHPLLPDTFNIEDQEYSFKQFLYNNSILLEHGLTQFVESFTRQIAGRVAGGRNVPIAVQAVAKASIDQSREMKYQSLNEYRKRFSLKPYTSFEELTGEKEMAAELKALYSDIDVMELYPALLVEKPRPDAIFGETMVELGAPFSLKGLMGNPICSPQYWKPSTFGGEVGFKIINTASIQSLICNNVKGCPFTSFNVQDPQPTKTATINASASHSRLDDINPTVLIKRRSTEL
Inhibitor
Name:
BDBM205485
Synonyms:
Cytotoxic conjugates, 2
Type:
Small organic molecule
Emp. Form.:
C77H92ClN3O22
Mol. Mass.:
1447.014
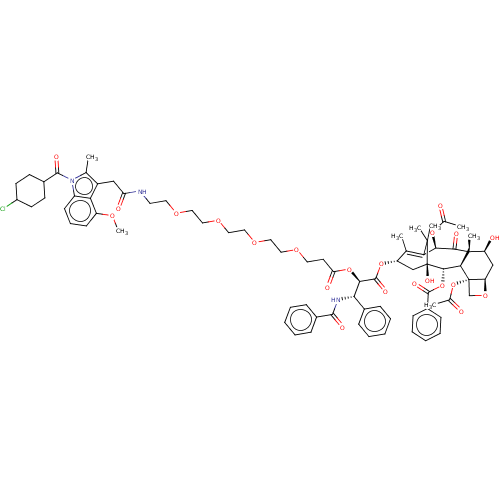
SMILES:
COc1cccc2n(C(=O)C3CCC(Cl)CC3)c(C)c(CC(=O)NCCOCCOCCOCCOCCC(=O)O[C@H]([C@@H](NC(=O)c3ccccc3)c3ccccc3)C(=O)O[C@H]3C[C@@]4(O)[C@@H](OC(=O)c5ccccc5)[C@@H]5[C@@]6(CO[C@@H]6C[C@H](O)[C@@]5(C)C(=O)[C@H](OC(C)=O)C(=C3C)C4(C)C)OC(C)=O)c12 |r,wU:75.79,63.66,79.82,81.86,83.89,87.93,wD:65.68,76.105,61.63,41.41,42.43,t:98,(7.3,-25.71,;8.33,-24.56,;9.84,-24.89,;10.31,-26.35,;11.82,-26.67,;12.85,-25.53,;12.37,-24.06,;13.14,-22.73,;14.68,-22.57,;15.3,-21.16,;15.58,-23.81,;14.96,-25.22,;15.86,-26.47,;17.39,-26.3,;18.3,-27.55,;18.02,-24.9,;17.11,-23.65,;12.11,-21.58,;12.43,-20.08,;10.71,-22.21,;9.37,-21.44,;9.37,-19.9,;10.71,-19.13,;8.04,-19.13,;8.04,-17.59,;6.71,-16.82,;6.71,-15.28,;5.37,-14.51,;5.37,-12.97,;4.04,-12.2,;4.04,-10.66,;2.71,-9.89,;2.71,-8.35,;1.37,-7.58,;1.37,-6.04,;.04,-5.27,;.04,-3.73,;-1.29,-2.96,;-1.29,-1.42,;.04,-.65,;-2.63,-.65,;-2.63,.89,;-3.96,1.66,;-3.96,3.2,;-5.3,3.97,;-6.63,3.2,;-5.3,5.51,;-3.96,6.28,;-3.96,7.82,;-5.3,8.59,;-6.63,7.82,;-6.63,6.28,;-5.3,.89,;-6.63,1.66,;-7.96,.89,;-7.96,-.65,;-6.63,-1.42,;-5.3,-.65,;-1.29,1.66,;-1.29,3.2,;.04,.89,;1.37,1.66,;2.46,.57,;3.79,1.34,;3.79,-.2,;5.33,1.34,;5.33,-.2,;4,-.97,;2.67,-.2,;4,-2.51,;2.67,-3.28,;2.67,-4.82,;4,-5.59,;5.33,-4.82,;5.33,-3.28,;6.42,2.43,;7.76,1.66,;8.85,.57,;10.18,1.34,;9.09,2.43,;9.09,3.97,;7.76,4.74,;7.76,6.28,;6.42,3.97,;6.42,5.51,;5.33,5.06,;5.92,6.48,;3.79,5.06,;3.21,6.48,;1.68,6.68,;.74,5.46,;1.09,8.11,;2.71,3.97,;1.37,3.2,;.04,3.97,;2.71,2.43,;3.48,3.76,;4.19,2.83,;7.76,.12,;9.09,-.65,;10.42,.12,;9.09,-2.19,;10.87,-23.74,)|