Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Cytochrome P450 3A4
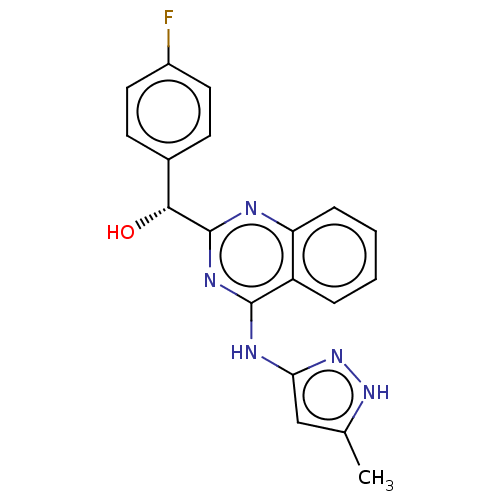
Ligand
BDBM214690
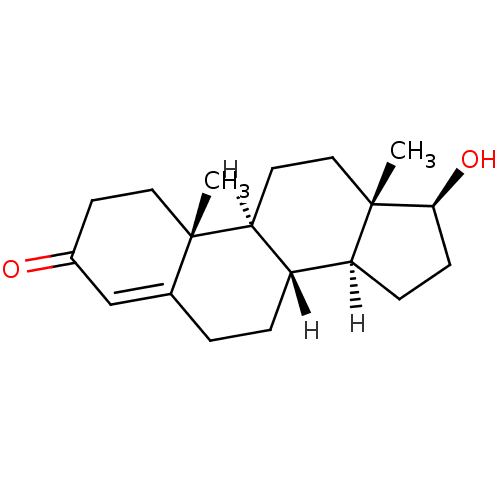
Substrate
BDBM8885
Meas. Tech.
CYP450 Inhibition Assay
Temperature
310.15±n/a K
IC50
14800±n/a nM
Comments
extracted
Citation
 Holladay, MW; Setti, E Optically active pyrazolylaminoquinazoline, and pharmaceutical compositions and methods of use thereof US Patent US9295672 Publication Date 3/29/2016
Holladay, MW; Setti, E Optically active pyrazolylaminoquinazoline, and pharmaceutical compositions and methods of use thereof US Patent US9295672 Publication Date 3/29/2016 More Info.:
Target
Name:
Cytochrome P450 3A4
Synonyms:
Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase
Type:
Enzyme
Mol. Mass.:
57349.57
Organism:
Homo sapiens (Human)
Description:
n/a
Residue:
503
Sequence:
MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMFDMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISIAEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYSMDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICVFPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSIIFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVVNETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFSKKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLGGLLQPEKPVVLKVESRDGTVSGA
Inhibitor
Name:
BDBM214690
Synonyms:
US9295672, (R)-(4-fluorophenyl)(4-((5-methyl-1H-pyrazol-3-yl)amino)quinazolin-2-yl)methanol
Type:
Small organic molecule
Emp. Form.:
C19H16FN5O
Mol. Mass.:
349.3616
SMILES:
Cc1cc(Nc2nc(nc3ccccc23)[C@H](O)c2ccc(F)cc2)n[nH]1
Substrate
Name:
BDBM8885
Synonyms:
(1S,2R,10R,11S,14S,15S)-14-hydroxy-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-6-en-5-one | 17beta-Hydroxyandrost-4-en-3-one | Testosterone | Testosterone, 1 | US9682960, Testosterone
Type:
Steroid
Emp. Form.:
C19H28O2
Mol. Mass.:
288.4244
SMILES:
[H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2=CC(=O)CC[C@]12C |r,t:18|