Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
GTPase KRas [G13C]
Ligand
BDBM591698
Substrate
n/a
Meas. Tech.
Biochemical Assay
IC50
5500±n/a nM
Citation
 Koltun, ES; Cregg, J; Gill, AL; Buckl, A; Liu, Y Ras inhibitors US Patent US11566007 Publication Date 1/31/2023
Koltun, ES; Cregg, J; Gill, AL; Buckl, A; Liu, Y Ras inhibitors US Patent US11566007 Publication Date 1/31/2023 More Info.:
Target
Name:
GTPase KRas [G13C]
Synonyms:
KRAS | KRAS2 | RASK2 | RASK_HUMAN
Type:
Enzyme Catalytic Domain
Mol. Mass.:
21702.19
Organism:
Homo sapiens (Human)
Description:
n/a
Residue:
189
Sequence:
MTEYKLVVVGAGCVGKSALTIQLIQNHFVDEYDPTIEDSYRKQVVIDGETCLLDILDTAGQEEYSAMRDQYMRTGEGFLCVFAINNTKSFEDIHHYREQIKRVKDSEDVPMVLVGNKCDLPSRTVDTKQAQDLARSYGIPFIETSAKTRQRVEDAFYTLVREIRQYRLKKISKEEKTPGCVKIKKCIIM
Inhibitor
Name:
BDBM591698
Synonyms:
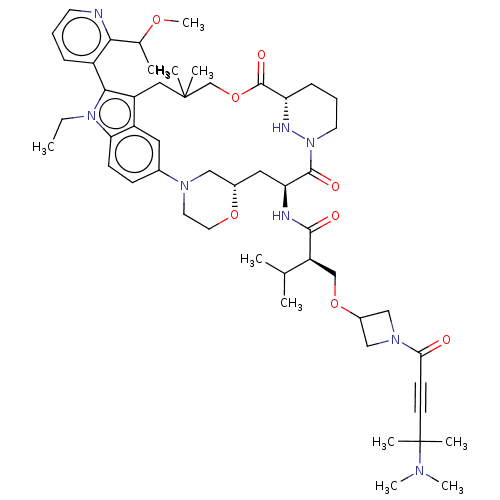
Synthesis of (2R)-2-(((1-(4-(dimethylamino)-4-methylpent-2-ynoyl)azetidin-3-yl)oxy)methyl)-N-((22S,63S,4S)-11-ethyl-12-(2-((S)-1-methoxyethyl)pyridin-3-yl)-10,10-dimethyl-5,7-dioxo-61,62,63,64,65,66-hexahydro-11H-8-oxa-2(4,2)-morpholina-1(5,3)-indola-6(1,3)-pyridazinacycloundecaphane-4-yl)-3-methylbutanamide | US11566007, Example A377
Type:
Small organic molecule
Emp. Form.:
C52H74N8O8
Mol. Mass.:
939.1928
SMILES:
CCn1c(c2CC(C)(C)COC(=O)[C@@H]3CCCN(N3)C(=O)[C@H](C[C@H]3CN(CCO3)c3ccc1c2c3)NC(=O)[C@@H](COC1CN(C1)C(=O)C#CC(C)(C)N(C)C)C(C)C)-c1cccnc1C(C)OC |r,wU:38.42,21.39,wD:13.18,23.29,(-9.59,-7.34,;-10.36,-6.01,;-9.59,-4.68,;-10.49,-3.43,;-9.59,-2.18,;-9.59,-.64,;-8.26,.13,;-7.49,-1.21,;-6.72,.13,;-8.11,1.95,;-6.78,2.72,;-6.78,4.26,;-8.11,5.03,;-5.44,5.03,;-5.44,6.57,;-4.11,7.34,;-2.78,6.57,;-2.78,5.03,;-4.11,4.26,;-1.44,4.26,;-.11,5.03,;-1.44,2.72,;-2.78,1.95,;-2.78,.41,;-4.11,-.36,;-4.11,-1.9,;-2.78,-2.67,;-1.44,-1.9,;-1.44,-.36,;-5.46,-2.66,;-5.46,-4.2,;-6.79,-4.97,;-8.12,-4.2,;-8.12,-2.66,;-6.79,-1.89,;-.11,1.95,;1.22,2.72,;1.22,4.26,;2.56,1.95,;3.89,2.72,;5.23,1.95,;6.56,2.72,;8.05,2.33,;8.45,3.81,;6.96,4.21,;9.78,4.58,;9.78,6.12,;11.11,3.81,;12.45,3.04,;13.78,2.27,;15.11,3.04,;13.78,3.81,;13.78,.73,;15.11,-.04,;12.45,-.04,;2.56,.41,;3.89,-.36,;1.22,-.36,;-12.03,-3.43,;-12.8,-4.76,;-14.34,-4.76,;-15.11,-3.43,;-14.34,-2.1,;-12.8,-2.1,;-12.03,-.76,;-10.49,-.76,;-12.8,.57,;-11.81,1.75,)|