Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Aryl hydrocarbon receptor
Ligand
BDBM75489
Substrate
n/a
Meas. Tech.
Luminescence-based cell-based high throughput dose response assay for activators of the Aryl Hydrocarbon Receptor (AHR)
EC50
3018±n/a nM
Citation
 PubChem, PC Luminescence-based cell-based high throughput dose response assay for activators of the Aryl Hydrocarbon Receptor (AHR) PubChem Bioassay (2010)[AID]
PubChem, PC Luminescence-based cell-based high throughput dose response assay for activators of the Aryl Hydrocarbon Receptor (AHR) PubChem Bioassay (2010)[AID] More Info.:
Target
Name:
Aryl hydrocarbon receptor
Synonyms:
AHR | AHR_HUMAN | BHLHE76 | Class E basic helix-loop-helix protein 76 | aryl hydrocarbon receptor precursor
Type:
PROTEIN
Mol. Mass.:
96143.77
Organism:
Homo sapiens (Human)
Description:
ChEMBL_1503828
Residue:
848
Sequence:
MNSSSANITYASRKRRKPVQKTVKPIPAEGIKSNPSKRHRDRLNTELDRLASLLPFPQDVINKLDKLSVLRLSVSYLRAKSFFDVALKSSPTERNGGQDNCRAANFREGLNLQEGEFLLQALNGFVLVVTTDALVFYASSTIQDYLGFQQSDVIHQSVYELIHTEDRAEFQRQLHWALNPSQCTESGQGIEEATGLPQTVVCYNPDQIPPENSPLMERCFICRLRCLLDNSSGFLAMNFQGKLKYLHGQKKKGKDGSILPPQLALFAIATPLQPPSILEIRTKNFIFRTKHKLDFTPIGCDAKGRIVLGYTEAELCTRGSGYQFIHAADMLYCAESHIRMIKTGESGMIVFRLLTKNNRWTWVQSNARLLYKNGRPDYIIVTQRPLTDEEGTEHLRKRNTKLPFMFTTGEAVLYEATNPFPAIMDPLPLRTKNGTSGKDSATTSTLSKDSLNPSSLLAAMMQQDESIYLYPASSTSSTAPFENNFFNESMNECRNWQDNTAPMGNDTILKHEQIDQPQDVNSFAGGHPGLFQDSKNSDLYSIMKNLGIDFEDIRHMQNEKFFRNDFSGEVDFRDIDLTDEILTYVQDSLSKSPFIPSDYQQQQSLALNSSCMVQEHLHLEQQQQHHQKQVVVEPQQQLCQKMKHMQVNGMFENWNSNQFVPFNCPQQDPQQYNVFTDLHGISQEFPYKSEMDSMPYTQNFISCNQPVLPQHSKCTELDYPMGSFEPSPYPTTSSLEDFVTCLQLPENQKHGLNPQSAIITPQTCYAGAVSMYQCQPEPQHTHVGQMQYNPVLPGQQAFLNKFQNGVLNETYPAELNNINNTQTTTHLQPLHHPSEARPFPDLTSSGFL
Inhibitor
Name:
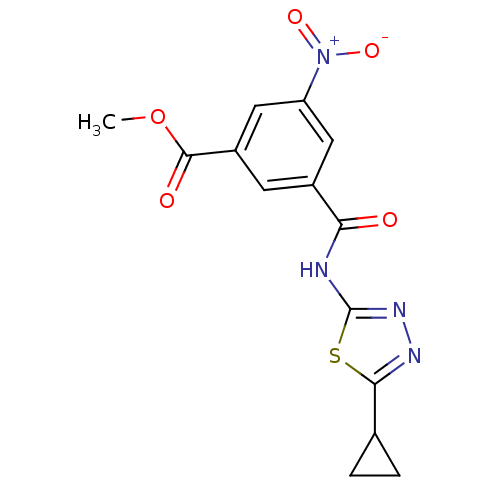
BDBM75489
Synonyms:
3-[(5-cyclopropyl-1,3,4-thiadiazol-2-yl)carbamoyl]-5-nitro-benzoic acid methyl ester | 3-[[(5-cyclopropyl-1,3,4-thiadiazol-2-yl)amino]-oxomethyl]-5-nitrobenzoic acid methyl ester | MLS000335393 | SMR000250142 | cid_4983475 | methyl 3-[(5-cyclopropyl-1,3,4-thiadiazol-2-yl)carbamoyl]-5-nitro-benzoate | methyl 3-[(5-cyclopropyl-1,3,4-thiadiazol-2-yl)carbamoyl]-5-nitrobenzoate
Type:
Small organic molecule
Emp. Form.:
C14H12N4O5S
Mol. Mass.:
348.334
SMILES:
COC(=O)c1cc(cc(c1)[N+]([O-])=O)C(=O)Nc1nnc(s1)C1CC1