Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Signal transducer and activator of transcription 5A
Ligand
BDBM50000029
Substrate
n/a
Meas. Tech.
ChEMBL_1704633 (CHEMBL4055866)
IC50
9400±n/a nM
Citation
More Info.:
Target
Name:
Signal transducer and activator of transcription 5A
Synonyms:
STA5A_HUMAN | STAT5 | STAT5A | Signal transducer and activator of transcription 5A | Transcription factor STAT5a (STAT5a)
Type:
Protein
Mol. Mass.:
90643.89
Organism:
Human
Description:
n/a
Residue:
794
Sequence:
MAGWIQAQQLQGDALRQMQVLYGQHFPIEVRHYLAQWIESQPWDAIDLDNPQDRAQATQLLEGLVQELQKKAEHQVGEDGFLLKIKLGHYATQLQKTYDRCPLELVRCIRHILYNEQRLVREANNCSSPAGILVDAMSQKHLQINQTFEELRLVTQDTENELKKLQQTQEYFIIQYQESLRIQAQFAQLAQLSPQERLSRETALQQKQVSLEAWLQREAQTLQQYRVELAEKHQKTLQLLRKQQTIILDDELIQWKRRQQLAGNGGPPEGSLDVLQSWCEKLAEIIWQNRQQIRRAEHLCQQLPIPGPVEEMLAEVNATITDIISALVTSTFIIEKQPPQVLKTQTKFAATVRLLVGGKLNVHMNPPQVKATIISEQQAKSLLKNENTRNECSGEILNNCCVMEYHQATGTLSAHFRNMSLKRIKRADRRGAESVTEEKFTVLFESQFSVGSNELVFQVKTLSLPVVVIVHGSQDHNATATVLWDNAFAEPGRVPFAVPDKVLWPQLCEALNMKFKAEVQSNRGLTKENLVFLAQKLFNNSSSHLEDYSGLSVSWSQFNRENLPGWNYTFWQWFDGVMEVLKKHHKPHWNDGAILGFVNKQQAHDLLINKPDGTFLLRFSDSEIGGITIAWKFDSPERNLWNLKPFTTRDFSIRSLADRLGDLSYLIYVFPDRPKDEVFSKYYTPVLAKAVDGYVKPQIKQVVPEFVNASADAGGSSATYMDQAPSPAVCPQAPYNMYPQNPDHVLDQDGEFDLDETMDVARHVEELLRRPMDSLDSRLSPPAGLFTSARGSLS
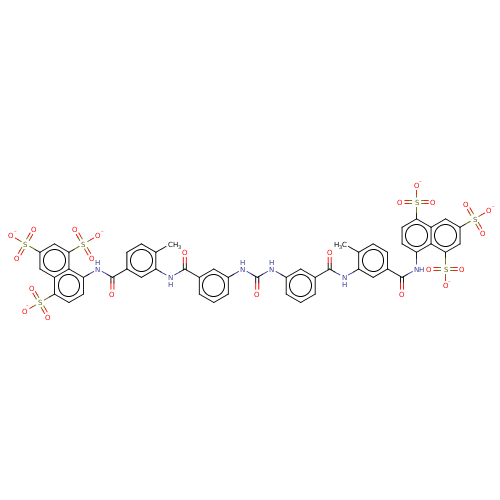
Inhibitor
Name:
BDBM50000029
Synonyms:
4-Methyl-8-{4-methyl-3-[3-(3-{3-[2-methyl-5-(4,6,8-trisulfo-naphthalen-1-ylcarbamoyl)-phenylcarbamoyl]-phenyl}-ureido)-benzoylamino]-benzoylamino}-naphthalene-1,3,5-trisulfonic acid; hexa-kis Sodium salt | 5,5',5''-[1,3,6-naphthalenetriyltris(sulfonylimino)]tris[1,3-benzenesulfonate analogue | CHEMBL265502 | SURAMIN HEXASODIUM | Suramin sodium | suramin | suramin Na
Type:
Small organic molecule
Emp. Form.:
C51H34N6O23S6
Mol. Mass.:
1291.235
SMILES:
Cc1ccc(cc1NC(=O)c1cccc(NC(=O)Nc2cccc(c2)C(=O)Nc2cc(ccc2C)C(=O)Nc2ccc(c3cc(cc(c23)S([O-])(=O)=O)S([O-])(=O)=O)S([O-])(=O)=O)c1)C(=O)Nc1ccc(c2cc(cc(c12)S([O-])(=O)=O)S([O-])(=O)=O)S([O-])(=O)=O