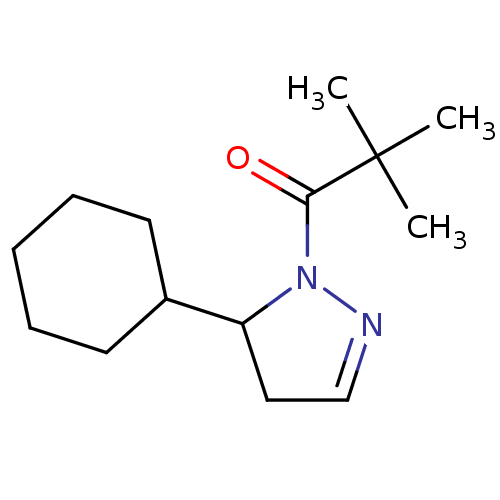
Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Receptor-interacting serine/threonine-protein kinase 1
Ligand
BDBM50513021
Substrate
n/a
Meas. Tech.
ChEMBL_1851533 (CHEMBL4352157)
IC50
794±n/a nM
Citation
 Harris, PA; Faucher, N; George, N; Eidam, PM; King, BW; White, GV; Anderson, NA; Bandyopadhyay, D; Beal, AM; Beneton, V; Berger, SB; Campobasso, N; Campos, S; Capriotti, CA; Cox, JA; Daugan, A; Donche, F; Fouchet, MH; Finger, JN; Geddes, B; Gough, PJ; Grondin, P; Hoffman, BL; Hoffman, SJ; Hutchinson, SE; Jeong, JU; Jigorel, E; Lamoureux, P; Leister, LK; Lich, JD; Mahajan, MK; Meslamani, J; Mosley, JE; Nagilla, R; Nassau, PM; Ng, SL; Ouellette, MT; Pasikanti, KK; Potvain, F; Reilly, MA; Rivera, EJ; Sautet, S; Schaeffer, MC; Sehon, CA; Sun, H; Thorpe, JH; Totoritis, RD; Ward, P; Wellaway, N; Wisnoski, DD; Woolven, JM; Bertin, J; Marquis, RW Discovery and Lead-Optimization of 4,5-Dihydropyrazoles as Mono-Kinase Selective, Orally Bioavailable and Efficacious Inhibitors of Receptor Interacting Protein 1 (RIP1) Kinase. J Med Chem 62:5096-5110 (2019) [PubMed] Article
Harris, PA; Faucher, N; George, N; Eidam, PM; King, BW; White, GV; Anderson, NA; Bandyopadhyay, D; Beal, AM; Beneton, V; Berger, SB; Campobasso, N; Campos, S; Capriotti, CA; Cox, JA; Daugan, A; Donche, F; Fouchet, MH; Finger, JN; Geddes, B; Gough, PJ; Grondin, P; Hoffman, BL; Hoffman, SJ; Hutchinson, SE; Jeong, JU; Jigorel, E; Lamoureux, P; Leister, LK; Lich, JD; Mahajan, MK; Meslamani, J; Mosley, JE; Nagilla, R; Nassau, PM; Ng, SL; Ouellette, MT; Pasikanti, KK; Potvain, F; Reilly, MA; Rivera, EJ; Sautet, S; Schaeffer, MC; Sehon, CA; Sun, H; Thorpe, JH; Totoritis, RD; Ward, P; Wellaway, N; Wisnoski, DD; Woolven, JM; Bertin, J; Marquis, RW Discovery and Lead-Optimization of 4,5-Dihydropyrazoles as Mono-Kinase Selective, Orally Bioavailable and Efficacious Inhibitors of Receptor Interacting Protein 1 (RIP1) Kinase. J Med Chem 62:5096-5110 (2019) [PubMed] Article More Info.:
Target
Name:
Receptor-interacting serine/threonine-protein kinase 1
Synonyms:
Cell death protein RIP | RIP | RIP-1 | RIP1 | RIPK1 | RIPK1_HUMAN | Receptor-interacting protein 1
Type:
Enzyme Catalytic Domain
Mol. Mass.:
75926.99
Organism:
Homo sapiens (Human)
Description:
Q13546
Residue:
671
Sequence:
MQPDMSLNVIKMKSSDFLESAELDSGGFGKVSLCFHRTQGLMIMKTVYKGPNCIEHNEALLEEAKMMNRLRHSRVVKLLGVIIEEGKYSLVMEYMEKGNLMHVLKAEMSTPLSVKGRIILEIIEGMCYLHGKGVIHKDLKPENILVDNDFHIKIADLGLASFKMWSKLNNEEHNELREVDGTAKKNGGTLYYMAPEHLNDVNAKPTEKSDVYSFAVVLWAIFANKEPYENAICEQQLIMCIKSGNRPDVDDITEYCPREIISLMKLCWEANPEARPTFPGIEEKFRPFYLSQLEESVEEDVKSLKKEYSNENAVVKRMQSLQLDCVAVPSSRSNSATEQPGSLHSSQGLGMGPVEESWFAPSLEHPQEENEPSLQSKLQDEANYHLYGSRMDRQTKQQPRQNVAYNREEERRRRVSHDPFAQQRPYENFQNTEGKGTAYSSAASHGNAVHQPSGLTSQPQVLYQNNGLYSSHGFGTRPLDPGTAGPRVWYRPIPSHMPSLHNIPVPETNYLGNTPTMPFSSLPPTDESIKYTIYNSTGIQIGAYNYMEIGGTSSSLLDSTNTNFKEEPAAKYQAIFDNTTSLTDKHLDPIRENLGKHWKNCARKLGFTQSQIDEIDHDYERDGLKEKVYQMLQKWVMREGIKGATVGKLAQALHQCSRIDLLSSLIYVSQN