Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Histamine H3 receptor
Ligand
BDBM50352092
Substrate
n/a
Meas. Tech.
ChEMBL_766551 (CHEMBL1827032)
Ki
2.3±n/a nM
Citation
 Labeeuw, O; Levoin, N; Poupardin-Olivier, O; Calmels, T; Ligneau, X; Berrebi-Bertrand, I; Robert, P; Lecomte, JM; Schwartz, JC; Capet, M Novel and highly potent histamine H3 receptor ligands. Part 2: exploring the cyclohexylamine-based series. Bioorg Med Chem Lett 21:5384-8 (2011) [PubMed] Article
Labeeuw, O; Levoin, N; Poupardin-Olivier, O; Calmels, T; Ligneau, X; Berrebi-Bertrand, I; Robert, P; Lecomte, JM; Schwartz, JC; Capet, M Novel and highly potent histamine H3 receptor ligands. Part 2: exploring the cyclohexylamine-based series. Bioorg Med Chem Lett 21:5384-8 (2011) [PubMed] Article More Info.:
Target
Name:
Histamine H3 receptor
Synonyms:
HH3R | HRH3_MOUSE | Hrh3
Type:
PROTEIN
Mol. Mass.:
48560.37
Organism:
Mus musculus
Description:
ChEMBL_988451
Residue:
445
Sequence:
MERAPPDGLMNASGALAGEAAAAGGARGFSAAWTAVLAALMALLIVATVLGNALVMLAFVADSSLRTQNNFFLLNLAISDFLVGAFCIPLYVPYVLTGRWTFGRGLCKLWLVVDYLLCASSVFNIVLISYDRFLSVTRAVSYRAQQGDTRRAVRKMALVWVLAFLLYGPAILSWEYLSGGSSIPEGHCYAEFFYNWYFLITASTLEFFTPFLSVTFFNLSIYLNIQRRTRLRLDGGREAGPEPPPDAQPSPPPAPPSCWGCWPKGHGEAMPLHRYGVGEAGPGVETGEAGLGGGSGGGAAASPTSSSGSSSRGTERPRSLKRGSKPSASSASLEKRMKMVSQSITQRFRLSRDKKVAKSLAIIVSIFGLCWAPYTLLMIIRAACHGHCVPDYWYETSFWLLWANSAVNPVLYPLCHYSFRRAFTKLLCPQKLKVQPHGSLEQCWK
Inhibitor
Name:
BDBM50352092
Synonyms:
CHEMBL1824239
Type:
Small organic molecule
Emp. Form.:
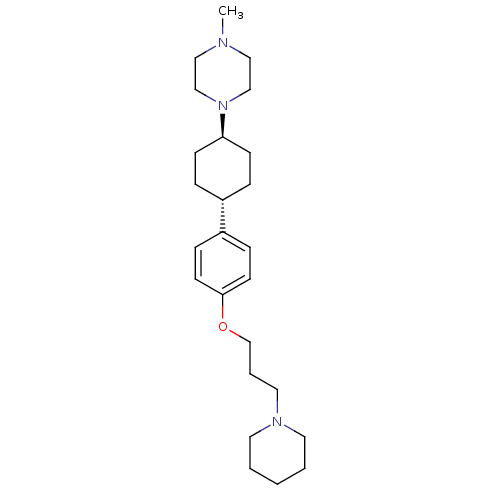
C25H41N3O
Mol. Mass.:
399.6125
SMILES:
CN1CCN(CC1)[C@H]1CC[C@@H](CC1)c1ccc(OCCCN2CCCCC2)cc1 |r,wU:7.7,wD:10.14,(38.82,-17.68,;37.28,-17.66,;36.49,-18.99,;34.95,-18.97,;34.2,-17.63,;34.98,-16.3,;36.52,-16.32,;32.66,-17.61,;31.88,-18.94,;30.34,-18.93,;29.58,-17.59,;30.35,-16.26,;31.9,-16.27,;28.04,-17.57,;27.26,-18.9,;25.73,-18.89,;24.96,-17.55,;23.42,-17.55,;22.65,-18.88,;21.11,-18.88,;20.34,-17.54,;18.8,-17.54,;18.04,-18.87,;16.5,-18.87,;15.72,-17.54,;16.5,-16.21,;18.04,-16.21,;25.73,-16.22,;27.27,-16.23,)|