Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Glycogen phosphorylase, liver form
Ligand
BDBM50149207
Substrate
n/a
Meas. Tech.
ChEMBL_1449752 (CHEMBL3378175)
IC50
6.0±n/a nM
Citation
 Parmenopoulou, V; Kantsadi, AL; Tsirkone, VG; Chatzileontiadou, DS; Manta, S; Zographos, SE; Molfeta, C; Archontis, G; Agius, L; Hayes, JM; Leonidas, DD; Komiotis, D Structure based inhibitor design targeting glycogen phosphorylase B. Virtual screening, synthesis, biochemical and biological assessment of novel N-acyl-ß-d-glucopyranosylamines. Bioorg Med Chem 22:4810-25 (2014) [PubMed] Article
Parmenopoulou, V; Kantsadi, AL; Tsirkone, VG; Chatzileontiadou, DS; Manta, S; Zographos, SE; Molfeta, C; Archontis, G; Agius, L; Hayes, JM; Leonidas, DD; Komiotis, D Structure based inhibitor design targeting glycogen phosphorylase B. Virtual screening, synthesis, biochemical and biological assessment of novel N-acyl-ß-d-glucopyranosylamines. Bioorg Med Chem 22:4810-25 (2014) [PubMed] Article More Info.:
Target
Name:
Glycogen phosphorylase, liver form
Synonyms:
Glycogen Phosphorylase (PYGL) | Glycogen Phosphorylase, liver form | Liver glycogen phosphorylase | PYGL | PYGL_HUMAN
Type:
Homodimer
Mol. Mass.:
97153.98
Organism:
Homo sapiens (Human)
Description:
Dimers associate into a tetramer to form the enzymatically active phosphorylase A.
Residue:
847
Sequence:
MAKPLTDQEKRRQISIRGIVGVENVAELKKSFNRHLHFTLVKDRNVATTRDYYFALAHTVRDHLVGRWIRTQQHYYDKCPKRVYYLSLEFYMGRTLQNTMINLGLQNACDEAIYQLGLDIEELEEIEEDAGLGNGGLGRLAACFLDSMATLGLAAYGYGIRYEYGIFNQKIRDGWQVEEADDWLRYGNPWEKSRPEFMLPVHFYGKVEHTNTGTKWIDTQVVLALPYDTPVPGYMNNTVNTMRLWSARAPNDFNLRDFNVGDYIQAVLDRNLAENISRVLYPNDNFFEGKELRLKQEYFVVAATLQDIIRRFKASKFGSTRGAGTVFDAFPDQVAIQLNDTHPALAIPELMRIFVDIEKLPWSKAWELTQKTFAYTNHTVLPEALERWPVDLVEKLLPRHLEIIYEINQKHLDRIVALFPKDVDRLRRMSLIEEEGSKRINMAHLCIVGSHAVNGVAKIHSDIVKTKVFKDFSELEPDKFQNKTNGITPRRWLLLCNPGLAELIAEKIGEDYVKDLSQLTKLHSFLGDDVFLRELAKVKQENKLKFSQFLETEYKVKINPSSMFDVQVKRIHEYKRQLLNCLHVITMYNRIKKDPKKLFVPRTVIIGGKAAPGYHMAKMIIKLITSVADVVNNDPMVGSKLKVIFLENYRVSLAEKVIPATDLSEQISTAGTEASGTGNMKFMLNGALTIGTMDGANVEMAEEAGEENLFIFGMRIDDVAALDKKGYEAKEYYEALPELKLVIDQIDNGFFSPKQPDLFKDIINMLFYHDRFKVFADYEAYVKCQDKVSQLYMNPKAWNTMVLKNIAASGKFSSDRTIKEYAQNIWNVEPSDLKISLSNESNKVNGN
Inhibitor
Name:
BDBM50149207
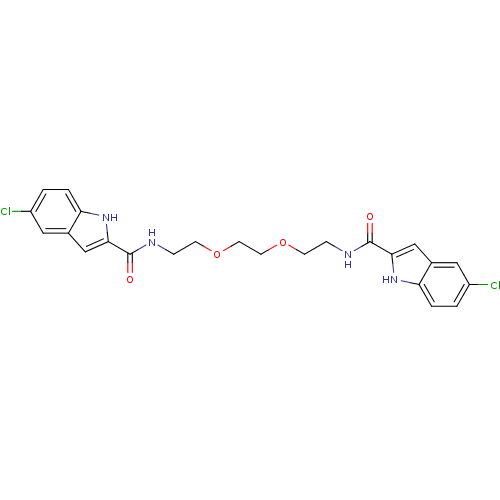
Synonyms:
2N-(2-{2-[2-(5-chloro-1H-2-indolylcarboxamido)ethoxy]ethoxy}ethyl)-5-chloro-1H-2-indolecarboxamide | BIS[5-CHLORO-1H-INDOL-2-YL-CARBONYL-AMINOETHYL]-ETHYLENE GLYCOL | CHEMBL434025
Type:
Small organic molecule
Emp. Form.:
C24H24Cl2N4O4
Mol. Mass.:
503.378
SMILES:
Clc1ccc2[nH]c(cc2c1)C(=O)NCCOCCOCCNC(=O)c1cc2cc(Cl)ccc2[nH]1