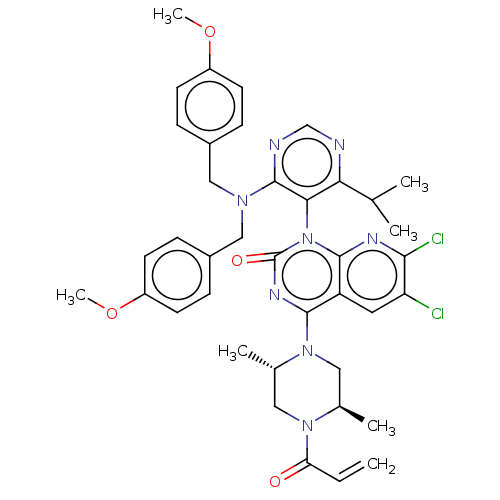
Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
GTPase KRas [1-169,G12C,C118A]
Ligand
BDBM544375
Substrate
n/a
Meas. Tech.
Coupled Nucleotide Exchange Assay
IC50
29.0±n/a nM
Citation
 Allen, JG; Lanman, BA; Chen, J; Reed, AB; Cee, VJ; Liu, L; Lopez, P; Wurz, RP; Nguyen, TT; Booker, S; Allen, JR; Chu-Moyer, M; Amegadzie, A; Chen, N; Goodman, C; Low, JD; Ma, VV; Minatti, AE; Nishimura, N; Pickrell, AJ; Wang, H; Shin, Y; Siegmund, AC; Yang, KC; Tamayo, NA; Walton, M; Xue, Q Substituted piperazines as KRAS G12C inhibitors US Patent US11285156 Publication Date 3/29/2022
Allen, JG; Lanman, BA; Chen, J; Reed, AB; Cee, VJ; Liu, L; Lopez, P; Wurz, RP; Nguyen, TT; Booker, S; Allen, JR; Chu-Moyer, M; Amegadzie, A; Chen, N; Goodman, C; Low, JD; Ma, VV; Minatti, AE; Nishimura, N; Pickrell, AJ; Wang, H; Shin, Y; Siegmund, AC; Yang, KC; Tamayo, NA; Walton, M; Xue, Q Substituted piperazines as KRAS G12C inhibitors US Patent US11285156 Publication Date 3/29/2022 More Info.:
Target
Name:
GTPase KRas [1-169,G12C,C118A]
Synonyms:
GTPase KRas | KRAS | KRAS2 | RASK2 | RASK_HUMAN
Type:
PROTEIN
Mol. Mass.:
19407.24
Organism:
Homo sapiens (Human)
Description:
P01116[1-169,G12C,C118A]
Residue:
169
Sequence:
MTEYKLVVVGACGVGKSALTIQLIQNHFVDEYDPTIEDSYRKQVVIDGETCLLDILDTAGQEEYSAMRDQYMRTGEGFLCVFAINNTKSFEDIHHYREQIKRVKDSEDVPMVLVGNKADLPSRTVDTKQAQDLARSYGIPFIETSAKTRQRVEDAFYTLVREIRQYRLK
Inhibitor
Name:
BDBM544375
Synonyms:
4-((2S,5R)-4-Acryloyl-2,5-dimethylpiperazin-1-yl)-7-(2-amino-6-fluorophenyl)-1-(4-amino-6-isopropylpyrimidin-5-yl)-6-chloropyrido[2,3-d]pyrimidin-2(1H)-one | US11285156, Ex.# 192
Type:
Small organic molecule
Emp. Form.:
C39H42Cl2N8O4
Mol. Mass.:
757.708
SMILES:
COc1ccc(CN(Cc2ccc(OC)cc2)c2ncnc(C(C)C)c2-n2c3nc(Cl)c(Cl)cc3c(nc2=O)N2C[C@@H](C)N(C[C@@H]2C)C(=O)C=C)cc1 |r,wU:45.50,wD:41.45,(5.93,-.55,;6.7,.79,;5.93,2.12,;4.39,2.12,;3.62,3.46,;4.39,4.79,;3.62,6.12,;2.08,6.12,;1.31,7.46,;2.08,8.79,;3.62,8.79,;4.39,10.12,;3.62,11.46,;4.39,12.79,;5.93,12.79,;2.08,11.46,;1.15,10.28,;1.31,4.79,;2.05,3.41,;1.28,2.07,;-.26,2.07,;-1.03,3.41,;-2.36,2.64,;-3.7,3.41,;-2.36,1.1,;-.26,4.74,;-1.57,7.07,;-.8,8.4,;.74,8.4,;1.51,9.74,;3.05,9.74,;.74,11.07,;1.51,12.41,;-.8,11.07,;-1.57,9.74,;-3.11,9.74,;-3.88,8.4,;-3.11,7.07,;-3.88,5.74,;-3.88,11.07,;-3.11,12.41,;-3.88,13.74,;-3.11,15.07,;-5.42,13.74,;-6.19,12.41,;-5.42,11.07,;-6.19,9.74,;-6.19,15.07,;-5.42,16.41,;-7.73,15.07,;-8.5,16.41,;5.93,4.79,;6.7,3.46,)|