Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit alpha
Ligand
BDBM587518
Substrate
n/a
Meas. Tech.
Kinase Assay
IC50
1.10±n/a nM
Citation
More Info.:
Target
Name:
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit alpha
Synonyms:
P3C2A_HUMAN | PI3K-C2-alpha | PIK3C2A | Phosphatidylinositol 4-phosphate 3-kinase C2 alpha (PIK3C2A) | Phosphatidylinositol-4-phosphate 3-kinase C2 domain-containing subunit alpha | Phosphoinositide 3-kinase-C2-alpha | PtdIns-3-kinase C2 subunit alpha
Type:
Protein
Mol. Mass.:
190704.50
Organism:
Homo sapiens (Human)
Description:
O00443
Residue:
1686
Sequence:
MAQISSNSGFKECPSSHPEPTRAKDVDKEEALQMEAEALAKLQKDRQVTDNQRGFELSSSTRKKAQVYNKQDYDLMVFPESDSQKRALDIDVEKLTQAELEKLLLDDSFETKKTPVLPVTPILSPSFSAQLYFRPTIQRGQWPPGLPGPSTYALPSIYPSTYSKQAAFQNGFNPRMPTFPSTEPIYLSLPGQSPYFSYPLTPATPFHPQGSLPIYRPVVSTDMAKLFDKIASTSEFLKNGKARTDLEITDSKVSNLQVSPKSEDISKFDWLDLDPLSKPKVDNVEVLDHEEEKNVSSLLAKDPWDAVLLEERSTANCHLERKVNGKSLSVATVTRSQSLNIRTTQLAKAQGHISQKDPNGTSSLPTGSSLLQEVEVQNEEMAAFCRSITKLKTKFPYTNHRTNPGYLLSPVTAQRNICGENASVKVSIDIEGFQLPVTFTCDVSSTVEIIIMQALCWVHDDLNQVDVGSYVLKVCGQEEVLQNNHCLGSHEHIQNCRKWDTEIRLQLLTFSAMCQNLARTAEDDETPVDLNKHLYQIEKPCKEAMTRHPVEELLDSYHNQVELALQIENQHRAVDQVIKAVRKICSALDGVETLAITESVKKLKRAVNLPRSKTADVTSLFGGEDTSRSSTRGSLNPENPVQVSINQLTAAIYDLLRLHANSGRSPTDCAQSSKSVKEAWTTTEQLQFTIFAAHGISSNWVSNYEKYYLICSLSHNGKDLFKPIQSKKVGTYKNFFYLIKWDELIIFPIQISQLPLESVLHLTLFGILNQSSGSSPDSNKQRKGPEALGKVSLPLFDFKRFLTCGTKLLYLWTSSHTNSVPGTVTKKGYVMERIVLQVDFPSPAFDIIYTTPQVDRSIIQQHNLETLENDIKGKLLDILHKDSSLGLSKEDKAFLWEKRYYCFKHPNCLPKILASAPNWKWVNLAKTYSLLHQWPALYPLIALELLDSKFADQEVRSLAVTWIEAISDDELTDLLPQFVQALKYEIYLNSSLVQFLLSRALGNIQIAHNLYWLLKDALHDVQFSTRYEHVLGALLSVGGKRLREELLKQTKLVQLLGGVAEKVRQASGSARQVVLQRSMERVQSFFQKNKCRLPLKPSLVAKELNIKSCSFFSSNAVPLKVTMVNADPMGEEINVMFKVGEDLRQDMLALQMIKIMDKIWLKEGLDLRMVIFKCLSTGRDRGMVELVPASDTLRKIQVEYGVTGSFKDKPLAEWLRKYNPSEEEYEKASENFIYSCAGCCVATYVLGICDRHNDNIMLRSTGHMFHIDFGKFLGHAQMFGSFKRDRAPFVLTSDMAYVINGGEKPTIRFQLFVDLCCQAYNLIRKQTNLFLNLLSLMIPSGLPELTSIQDLKYVRDALQPQTTDAEATIFFTRLIESSLGSIATKFNFFIHNLAQLRFSGLPSNDEPILSFSPKTYSFRQDGRIKEVSVFTYHKKYNPDKHYIYVVRILREGQIEPSFVFRTFDEFQELHNKLSIIFPLWKLPGFPNRMVLGRTHIKDVAAKRKIELNSYLQSLMNASTDVAECDLVCTFFHPLLRDEKAEGIARSADAGSFSPTPGQIGGAVKLSISYRNGTLFIMVMHIKDLVTEDGADPNPYVKTYLLPDNHKTSKRKTKISRKTRNPTFNEMLVYSGYSKETLRQRELQLSVLSAESLRENFFLGGVTLPLKDFNLSKETVKWYQLTAATYL
Inhibitor
Name:
BDBM587518
Synonyms:
US11534443, Example 18-1
Type:
Small organic molecule
Emp. Form.:
C28H29F2N5O5S
Mol. Mass.:
585.622
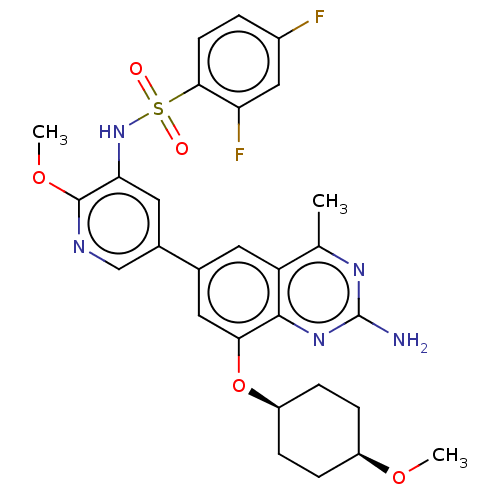
SMILES:
CO[C@H]1CC[C@H](CC1)Oc1cc(cc2c(C)nc(N)nc12)-c1cnc(OC)c(NS(=O)(=O)c2ccc(F)cc2F)c1 |r,wU:5.8,2.1,(-7.44,-11.71,;-6.1,-12.48,;-4.77,-11.71,;-4.77,-10.17,;-3.44,-9.4,;-2.1,-10.17,;-2.1,-11.71,;-3.44,-12.48,;-.77,-9.4,;-.77,-7.86,;.56,-7.09,;.56,-5.55,;-.77,-4.78,;-2.1,-5.55,;-3.44,-4.78,;-3.44,-3.24,;-4.77,-5.55,;-4.77,-7.09,;-6.1,-7.86,;-3.44,-7.86,;-2.1,-7.09,;1.9,-4.78,;1.9,-3.24,;3.23,-2.47,;4.61,-3.21,;5.95,-2.44,;5.95,-.9,;4.56,-4.78,;5.9,-5.55,;5.9,-7.09,;7.44,-7.09,;4.36,-7.09,;5.9,-8.63,;7.23,-9.4,;7.23,-10.94,;5.9,-11.71,;5.9,-13.25,;4.56,-10.94,;4.56,-9.4,;3.23,-8.63,;3.23,-5.55,)|