Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Galectin-3
Ligand
BDBM620311
Substrate
n/a
Meas. Tech.
Evaluation of Compound Inhibitory Activity (IC50)
IC50
15.0±n/a nM
Citation
 BOLLLI, M; GATFIELD, J; GRISOSTOMI, C; REMEN, L; SAGER, C; ZUMBRUNN, C SPIRO DERIVATIVES OF ALPHA-D-GALACTOPYRANOSIDES US Patent US20230295182 Publication Date 9/21/2023
BOLLLI, M; GATFIELD, J; GRISOSTOMI, C; REMEN, L; SAGER, C; ZUMBRUNN, C SPIRO DERIVATIVES OF ALPHA-D-GALACTOPYRANOSIDES US Patent US20230295182 Publication Date 9/21/2023 More Info.:
Target
Name:
Galectin-3
Synonyms:
LEG3_HUMAN | LGALS3 | MAC2
Type:
Enzyme
Mol. Mass.:
26156.54
Organism:
Homo sapiens (Human)
Description:
P17931
Residue:
250
Sequence:
MADNFSLHDALSGSGNPNPQGWPGAWGNQPAGAGGYPGASYPGAYPGQAPPGAYPGQAPPGAYPGAPGAYPGAPAPGVYPGPPSGPGAYPSSGQPSATGAYPATGPYGAPAGPLIVPYNLPLPGGVVPRMLITILGTVKPNANRIALDFQRGNDVAFHFNPRFNENNRRVIVCNTKLDNNWGREERQSVFPFESGKPFKIQVLVEPDHFKVAVNDAHLLQYNHRVKKLNEISKLGISGDIDLTSASYTMI
Inhibitor
Name:
BDBM620311
Synonyms:
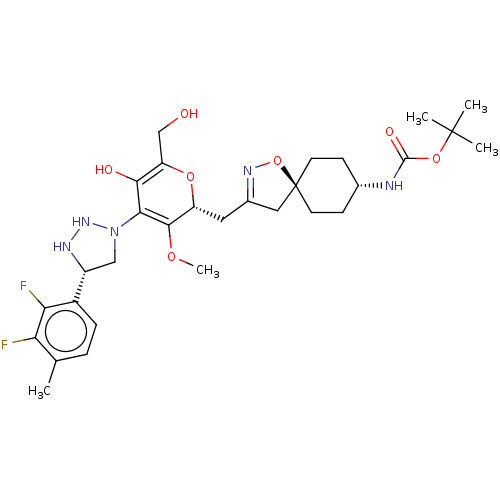
US20230295182, Example 1.43 | US20230295182, Example 1.44 | tert-butyl ((5r,8R)-3-(((2R,3R,4S,5R,6R)-4-(4-(2,3-difluoro-4-methylphenyl)- 1H-1,2,3-triazol-1-yl)-5-hydroxy-6-(hydroxymethyl)-3-methoxytetrahydro-2H- pyran-2-yl)methyl)-1-oxa-2-azaspiro[4.5]dec-2-en-8-yl)carbamate
Type:
Small organic molecule
Emp. Form.:
C30H41F2N5O7
Mol. Mass.:
621.6726
SMILES:
COC1=C(N2C[C@@H](NN2)c2ccc(C)c(F)c2F)C(O)=C(CO)O[C@@H]1CC1=NO[C@@]2(C1)CC[C@@H](CC2)NC(=O)OC(C)(C)C |wU:29.31,6.9,24.27,wD:33.40,c:2,t:21,29,(-1.33,-.88,;-2.23,-2.13,;-3.76,-1.97,;-4.39,-.56,;-3.48,.69,;-1.94,.69,;-1.47,2.15,;-2.71,3.06,;-3.96,2.15,;-0,2.63,;1.14,1.6,;2.61,2.07,;2.93,3.58,;4.39,4.05,;1.78,4.61,;2.1,6.11,;.32,4.13,;-.83,5.16,;-5.92,-.4,;-6.55,1.01,;-6.83,-1.65,;-8.36,-1.48,;-9.26,-2.73,;-6.2,-3.05,;-4.67,-3.21,;-4.04,-4.62,;-2.51,-4.78,;-1.74,-6.11,;-.23,-5.79,;-.07,-4.26,;-1.48,-3.64,;-.07,-2.72,;1.26,-1.95,;2.59,-2.72,;2.59,-4.26,;1.26,-5.03,;3.93,-1.95,;5.26,-2.72,;5.26,-4.26,;6.6,-1.95,;7.93,-2.72,;9.26,-1.95,;9.26,-3.49,;7.93,-4.26,)|