Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Histone deacetylase 4
Ligand
BDBM293896
Substrate
n/a
Meas. Tech.
Inhibition Assay
IC50
30.0±n/a nM
Citation
 Dominguez, C; Muñoz-Sanjuán, I; Maillard, M; Luckhurst, CA; Jarvis, RE; Bürli, RW; Allen, DR; Haughan, AF; Breccia, P; Vater, HD; Stott, AJ; Penrose, SD; Wall, M; Saville-Stones, EA; Wishart, G; Hughes, SJ Histone deacetylase inhibitors and compositions and methods of use thereof US Patent US9617259 Publication Date 4/11/2017
Dominguez, C; Muñoz-Sanjuán, I; Maillard, M; Luckhurst, CA; Jarvis, RE; Bürli, RW; Allen, DR; Haughan, AF; Breccia, P; Vater, HD; Stott, AJ; Penrose, SD; Wall, M; Saville-Stones, EA; Wishart, G; Hughes, SJ Histone deacetylase inhibitors and compositions and methods of use thereof US Patent US9617259 Publication Date 4/11/2017 More Info.:
Target
Name:
Histone deacetylase 4
Synonyms:
Cereblon/Histone deacetylase 4 | HD4 | HDAC4 | HDAC4_HUMAN | Histone acetylase 4(HDAC4) | Human HDAC4 | KIAA0288
Type:
Enzyme
Mol. Mass.:
119049.39
Organism:
Homo sapiens (Human)
Description:
P56524
Residue:
1084
Sequence:
MSSQSHPDGLSGRDQPVELLNPARVNHMPSTVDVATALPLQVAPSAVPMDLRLDHQFSLPVAEPALREQQLQQELLALKQKQQIQRQILIAEFQRQHEQLSRQHEAQLHEHIKQQQEMLAMKHQQELLEHQRKLERHRQEQELEKQHREQKLQQLKNKEKGKESAVASTEVKMKLQEFVLNKKKALAHRNLNHCISSDPRYWYGKTQHSSLDQSSPPQSGVSTSYNHPVLGMYDAKDDFPLRKTASEPNLKLRSRLKQKVAERRSSPLLRRKDGPVVTALKKRPLDVTDSACSSAPGSGPSSPNNSSGSVSAENGIAPAVPSIPAETSLAHRLVAREGSAAPLPLYTSPSLPNITLGLPATGPSAGTAGQQDAERLTLPALQQRLSLFPGTHLTPYLSTSPLERDGGAAHSPLLQHMVLLEQPPAQAPLVTGLGALPLHAQSLVGADRVSPSIHKLRQHRPLGRTQSAPLPQNAQALQHLVIQQQHQQFLEKHKQQFQQQQLQMNKIIPKPSEPARQPESHPEETEEELREHQALLDEPYLDRLPGQKEAHAQAGVQVKQEPIESDEEEAEPPREVEPGQRQPSEQELLFRQQALLLEQQRIHQLRNYQASMEAAGIPVSFGGHRPLSRAQSSPASATFPVSVQEPPTKPRFTTGLVYDTLMLKHQCTCGSSSSHPEHAGRIQSIWSRLQETGLRGKCECIRGRKATLEELQTVHSEAHTLLYGTNPLNRQKLDSKKLLGSLASVFVRLPCGGVGVDSDTIWNEVHSAGAARLAVGCVVELVFKVATGELKNGFAVVRPPGHHAEESTPMGFCYFNSVAVAAKLLQQRLSVSKILIVDWDVHHGNGTQQAFYSDPSVLYMSLHRYDDGNFFPGSGAPDEVGTGPGVGFNVNMAFTGGLDPPMGDAEYLAAFRTVVMPIASEFAPDVVLVSSGFDAVEGHPTPLGGYNLSARCFGYLTKQLMGLAGGRIVLALEGGHDLTAICDASEACVSALLGNELDPLPEKVLQQRPNANAVRSMEKVMEIHSKYWRCLQRTTSTAGRSLIEAQTCENEEAETVTAMASLSVGVKPAEKRPDEEPMEEEPPL
Inhibitor
Name:
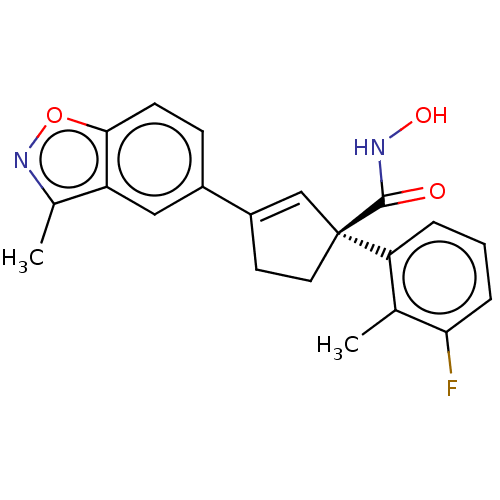
BDBM293896
Synonyms:
(S)-1-(3-Fluoro-2- methylphenyl)-N- hydroxy-3-(3- methylbenzo[d]isoxazol- 5-yl)cyclopent-2- enecarboxamide | US10106535, Example 22 | US9617259, 22
Type:
Small organic molecule
Emp. Form.:
C21H19FN2O3
Mol. Mass.:
366.3856
SMILES:
Cc1noc2ccc(cc12)C1=C[C@](CC1)(C(=O)NO)c1cccc(F)c1C |r,t:12|