Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Rho GTPase-activating protein 35
Ligand
BDBM13775
Substrate
n/a
Meas. Tech.
Glucocorticoid Receptor Transactivation Potencies for Cortisol and 17-ester Derivatives
EC50
41.6±n/a nM
Citation
 Edelman, JL; Nehme, A Pharmaceutical compositions and methods of use of 4-pregenen-11β-17-21-triol-3,20-dione derivatives US Patent US10188667 Publication Date 1/29/2019
Edelman, JL; Nehme, A Pharmaceutical compositions and methods of use of 4-pregenen-11β-17-21-triol-3,20-dione derivatives US Patent US10188667 Publication Date 1/29/2019 More Info.:
Target
Name:
Rho GTPase-activating protein 35
Synonyms:
ARHGAP35 | GRF-1 | GRF1 | GRLF1 | Glucocorticoid receptor DNA-binding factor 1 | Glucocorticoid receptor repression factor 1 | KIAA1722 | P190A | RHG35_HUMAN | Rho GAP p190A | p190-A | p190ARHOGAP
Type:
Enzyme Catalytic Domain
Mol. Mass.:
170514.61
Organism:
Homo sapiens (Human)
Description:
Q9NRY4
Residue:
1499
Sequence:
MMMARKQDVRIPTYNISVVGLSGTEKEKGQCGIGKSCLCNRFVRPSADEFHLDHTSVLSTSDFGGRVVNNDHFLYWGEVSRSLEDCVECKMHIVEQTEFIDDQTFQPHRSTALQPYIKRAAATKLASAEKLMYFCTDQLGLEQDFEQKQMPDGKLLVDGFLLGIDVSRGMNRNFDDQLKFVSNLYNQLAKTKKPIVVVLTKCDEGVERYIRDAHTFALSKKNLQVVETSARSNVNVDLAFSTLVQLIDKSRGKTKIIPYFEALKQQSQQIATAKDKYEWLVSRIVKNHNENWLSVSRKMQASPEYQDYVYLEGTQKAKKLFLQHIHRLKHEHIERRRKLYLAALPLAFEALIPNLDEIDHLSCIKAKKLLETKPEFLKWFVVLEETPWDATSHIDNMENERIPFDLMDTVPAEQLYEAHLEKLRNERKRVEMRRAFKENLETSPFITPGKPWEEARSFIMNEDFYQWLEESVYMDIYGKHQKQIIDKAKEEFQELLLEYSELFYELELDAKPSKEKMGVIQDVLGEEQRFKALQKLQAERDALILKHIHFVYHPTKETCPSCPACVDAKIEHLISSRFIRPSDRNQKNSLSDPNIDRINLVILGKDGLARELANEIRALCTNDDKYVIDGKMYELSLRPIEGNVRLPVNSFQTPTFQPHGCLCLYNSKESLSYVVESIEKSRESTLGRRDNHLVHLPLTLILVNKRGDTSGETLHSLIQQGQQIASKLQCVFLDPASAGIGYGRNINEKQISQVLKGLLDSKRNLNLVSSTASIKDLADVDLRIVMCLMCGDPFSADDILFPVLQSQTCKSSHCGSNNSVLLELPIGLHKKRIELSVLSYHSSFSIRKSRLVHGYIVFYSAKRKASLAMLRAFLCEVQDIIPIQLVALTDGAVDVLDNDLSREQLTEGEEIAQEIDGRFTSIPCSQPQHKLEIFHPFFKDVVEKKNIIEATHMYDNAAEACSTTEEVFNSPRAGSPLCNSNLQDSEEDIEPSYSLFREDTSLPSLSKDHSKLSMELEGNDGLSFIMSNFESKLNNKVPPPVKPKPPVHFEITKGDLSYLDQGHRDGQRKSVSSSPWLPQDGFDPSDYAEPMDAVVKPRNEEENIYSVPHDSTQGKIITIRNINKAQSNGSGNGSDSEMDTSSLERGRKVSIVSKPVLYRTRCTRLGRFASYRTSFSVGSDDELGPIRKKEEDQASQGYKGDNAVIPYETDEDPRRRNILRSLRRNTKKPKPKPRPSITKATWESNYFGVPLTTVVTPEKPIPIFIERCIEYIEATGLSTEGIYRVSGNKSEMESLQRQFDQDHNLDLAEKDFTVNTVAGAMKSFFSELPDPLVPYNMQIDLVEAHKINDREQKLHALKEVLKKFPKENHEVFKYVISHLNKVSHNNKVNLMTSENLSICFWPTLMRPDFSTMDALTATRTYQTIIELFIQQCPFFFYNRPITEPPGARPSSPSAVASTVPFLTSTPVTSQPSPPQSPPPTPQSPMQPLLPSQLQAEHTL
Inhibitor
Name:
BDBM13775
Synonyms:
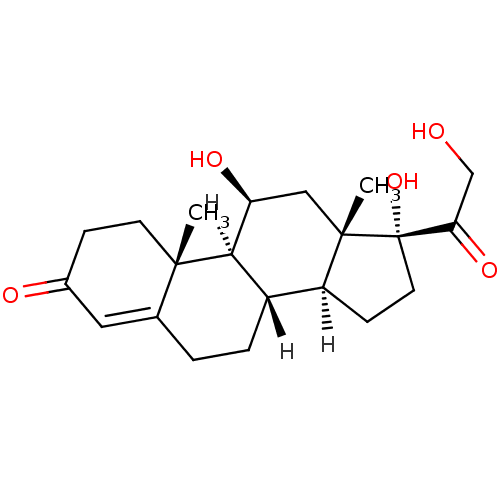
(1S,2R,10S,11S,14R,15S,17S)-14,17-dihydroxy-14-(2-hydroxyacetyl)-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-6-en-5-one | 11beta,17alpha,21-Trihydroxy-4-pregnene-3,20-dione | 3H-cortisol | HYDROCORTISONE | US10188667, Example 00023 | [3H]cortisol | cortisol
Type:
Steroid
Emp. Form.:
C21H30O5
Mol. Mass.:
362.4599
SMILES:
[H][C@@]12CC[C@](O)(C(=O)CO)[C@@]1(C)C[C@H](O)[C@@]1([H])[C@@]2([H])CCC2=CC(=O)CC[C@]12C |t:23|