Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Fatty acid synthase [2202-2509]
Ligand
BDBM24577
Substrate
BDBM24568
Meas. Tech.
Fluorogenic Assay for Detection of FASTE Inhibition
pH
7.4±n/a
Temperature
310.15±n/a K
IC50
370±60 nM
Citation
 Richardson, RD; Ma, G; Oyola, Y; Zancanella, M; Knowles, LM; Cieplak, P; Romo, D; Smith, JW Synthesis of novel beta-lactone inhibitors of fatty acid synthase. J Med Chem 51:5285-96 (2008) [PubMed] Article
Richardson, RD; Ma, G; Oyola, Y; Zancanella, M; Knowles, LM; Cieplak, P; Romo, D; Smith, JW Synthesis of novel beta-lactone inhibitors of fatty acid synthase. J Med Chem 51:5285-96 (2008) [PubMed] Article Target
Name:
Fatty acid synthase [2202-2509]
Synonyms:
FAS | FASN | FAS_HUMAN | Fatty Acid Synthase
Type:
Thioesterase domain
Mol. Mass.:
33927.11
Organism:
Homo sapiens (Human)
Description:
The recombinant thioesterase domain (residues 2202-2509) of FAS was cloned and expressed in Escheria coli. The thioesterase was purified by Ni-affinity chromatography, and analyzed for activity and inhibition by Orlistat.
Residue:
308
Sequence:
CPTPKEDGLAQQQTQLNLRSLLVNPEGPTLMRLNSVQSSERPLFLVHPIEGSTTVFHSLASRLSIPTYGLQCTRAAPLDSIHSLAAYYIDCIRQVQPEGPYRVAGYSYGACVAFEMCSQLQAQQSPAPTHNSLFLFDGSPTYVLAYTQSYRAKLTPGCEAEAETEAICFFVQQFTDMEHNRVLEALLPLKGLEERVAAAVDLIIKSHQGLDRQELSFAARSFYYKLRAAEQYTPKAKYHGNVMLLRAKTGGAYGEDLGADYNLSQVCDGKVSVHVIEGDHRTLLEGSGLESIISIIHSSLAEPRVSVR
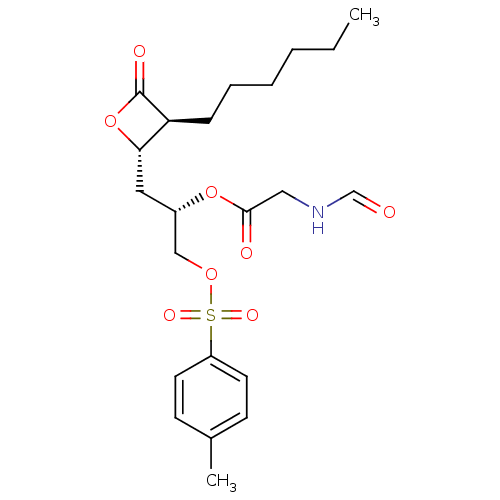
Inhibitor
Name:
BDBM24577
Synonyms:
(2S)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]-3-{[(4-methylbenzene)sulfonyl]oxy}propan-2-yl 2-formamidoacetate | Orlistat derivative, 17e
Type:
Small organic molecule
Emp. Form.:
C22H31NO8S
Mol. Mass.:
469.548
SMILES:
CCCCCC[C@H]1[C@H](C[C@@H](COS(=O)(=O)c2ccc(C)cc2)OC(=O)CNC=O)OC1=O |r|