Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Epstein-Barr nuclear antigen 1
Ligand
BDBM62434
Substrate
n/a
Meas. Tech.
Fluorescence polarization-based biochemical high throughput dose response assay for inhibitors of the Epstein-Barr virus nuclear antigen 1 (EBNA-1)
IC50
5725±n/a nM
Citation
 PubChem, PC Fluorescence polarization-based biochemical high throughput dose response assay for inhibitors of the Epstein-Barr virus nuclear antigen 1 (EBNA-1) PubChem Bioassay (2010)[AID]
PubChem, PC Fluorescence polarization-based biochemical high throughput dose response assay for inhibitors of the Epstein-Barr virus nuclear antigen 1 (EBNA-1) PubChem Bioassay (2010)[AID] More Info.:
Target
Name:
Epstein-Barr nuclear antigen 1
Synonyms:
EBNA-1 protein | EBNA1 | EBNA1_EBVB9 | Epstein-Barr virus protease (EBV Pr)
Type:
Enzyme Catalytic Domain
Mol. Mass.:
56444.81
Organism:
Human herpesvirus 4
Description:
gi_23893623
Residue:
641
Sequence:
MSDEGPGTGPGNGLGEKGDTSGPEGSGGSGPQRRGGDNHGRGRGRGRGRGGGRPGAPGGSGSGPRHRDGVRRPQKRPSCIGCKGTHGGTGAGAGAGGAGAGGAGAGGGAGAGGGAGGAGGAGGAGAGGGAGAGGGAGGAGGAGAGGGAGAGGGAGGAGAGGGAGGAGGAGAGGGAGAGGGAGGAGAGGGAGGAGGAGAGGGAGAGGAGGAGGAGAGGAGAGGGAGGAGGAGAGGAGAGGAGAGGAGAGGAGGAGAGGAGGAGAGGAGGAGAGGGAGGAGAGGGAGGAGAGGAGGAGAGGAGGAGAGGAGGAGAGGGAGAGGAGAGGGGRGRGGSGGRGRGGSGGRGRGGSGGRRGRGRERARGGSRERARGRGRGRGEKRPRSPSSQSSSSGSPPRRPPPGRRPFFHPVGEADYFEYHQEGGPDGEPDVPPGAIEQGPADDPGEGPSTGPRGQGDGGRRKKGGWFGKHRGQGGSNPKFENIAEGLRALLARSHVERTTDEGTWVAGVFVYGGSKTSLYNLRRGTALAIPQCRLTPLSRLPFGMAPGPGPQPGPLRESIVCYFMVFLQTHIFAEVLKDAIKDLVMTKPAPTCNIRVTVCSFDDGVDLPPWFPPMVEGAAAEGDDGDDGDEGGDGDEGEEGQE
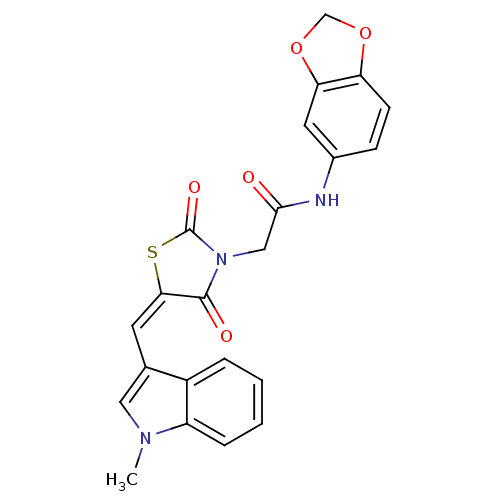
Inhibitor
Name:
BDBM62434
Synonyms:
MLS000325007 | N-(1,3-benzodioxol-5-yl)-2-[(5E)-2,4-diketo-5-[(1-methylindol-3-yl)methylene]thiazolidin-3-yl]acetamide | N-(1,3-benzodioxol-5-yl)-2-[(5E)-5-[(1-methyl-3-indolyl)methylidene]-2,4-dioxo-3-thiazolidinyl]acetamide | N-(1,3-benzodioxol-5-yl)-2-[(5E)-5-[(1-methylindol-3-yl)methylidene]-2,4-bis(oxidanylidene)-1,3-thiazolidin-3-yl]ethanamide | N-(1,3-benzodioxol-5-yl)-2-[(5E)-5-[(1-methylindol-3-yl)methylidene]-2,4-dioxo-1,3-thiazolidin-3-yl]acetamide | N-(1,3-benzodioxol-5-yl)-2-{5-[(1-methyl-1H-indol-3-yl)methylene]-2,4-dioxo-1,3-thiazolidin-3-yl}acetamide | SMR000160985 | cid_1229516
Type:
Small organic molecule
Emp. Form.:
C22H17N3O5S
Mol. Mass.:
435.452
SMILES:
Cn1cc(\C=C2\SC(=O)N(CC(=O)Nc3ccc4OCOc4c3)C2=O)c2ccccc12