Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Intestinal-type alkaline phosphatase
Ligand
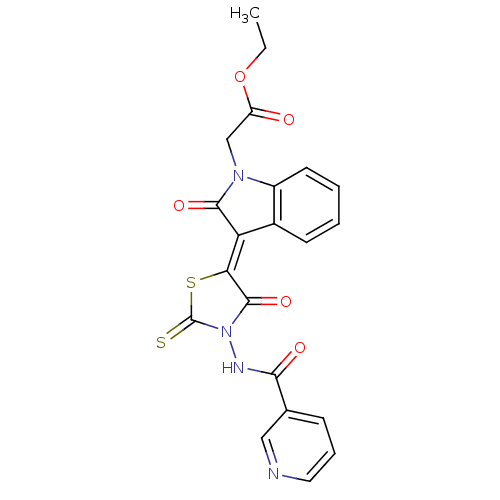
BDBM76202
Substrate
n/a
Meas. Tech.
Dose Response confirmation of uHTS activators of Mouse Intestinal Alkaline Phosphatase using Human Intestinal Alkaline Phosphatase
EC50
25500±n/a nM
Citation
 PubChem, PC Dose Response confirmation of uHTS activators of Mouse Intestinal Alkaline Phosphatase using Human Intestinal Alkaline Phosphatase PubChem Bioassay (2010)[AID]
PubChem, PC Dose Response confirmation of uHTS activators of Mouse Intestinal Alkaline Phosphatase using Human Intestinal Alkaline Phosphatase PubChem Bioassay (2010)[AID] More Info.:
Target
Name:
Intestinal-type alkaline phosphatase
Synonyms:
ALPI | Alkaline phosphatase, intestinal | Intestinal alkaline phosphatase | PPBI_HUMAN
Type:
PROTEIN
Mol. Mass.:
56804.94
Organism:
Homo sapiens (Human)
Description:
ChEMBL_1278525
Residue:
528
Sequence:
MQGPWVLLLLGLRLQLSLGVIPAEEENPAFWNRQAAEALDAAKKLQPIQKVAKNLILFLGDGLGVPTVTATRILKGQKNGKLGPETPLAMDRFPYLALSKTYNVDRQVPDSAATATAYLCGVKANFQTIGLSAAARFNQCNTTRGNEVISVMNRAKQAGKSVGVVTTTRVQHASPAGTYAHTVNRNWYSDADMPASARQEGCQDIATQLISNMDIDVILGGGRKYMFPMGTPDPEYPADASQNGIRLDGKNLVQEWLAKHQGAWYVWNRTELMQASLDQSVTHLMGLFEPGDTKYEIHRDPTLDPSLMEMTEAALRLLSRNPRGFYLFVEGGRIDHGHHEGVAYQALTEAVMFDDAIERAGQLTSEEDTLTLVTADHSHVFSFGGYTLRGSSIFGLAPSKAQDSKAYTSILYGNGPGYVFNSGVRPDVNESESGSPDYQQQAAVPLSSETHGGEDVAVFARGPQAHLVHGVQEQSFVAHVMAFAACLEPYTACDLAPPACTTDAAHPVAASLPLLAGTLLLLGASAAP
Inhibitor
Name:
BDBM76202
Synonyms:
(2-Oxo-3-{4-oxo-3-[(pyridine-3-carbonyl)-amino]-2-thioxo-thiazolidin-5-ylidene}-2,3-dihydro-indol-1-yl)-acetic acid ethyl ester | 2-[(3Z)-2-keto-3-(4-keto-3-nicotinamido-2-thioxo-thiazolidin-5-ylidene)indolin-1-yl]acetic acid ethyl ester | 2-[(3Z)-2-oxo-3-[4-oxo-3-[[oxo(3-pyridinyl)methyl]amino]-2-sulfanylidene-5-thiazolidinylidene]-1-indolyl]acetic acid ethyl ester | MLS001211884 | SMR000515674 | cid_1631117 | ethyl 2-[(3Z)-2-oxidanylidene-3-[4-oxidanylidene-3-(pyridin-3-ylcarbonylamino)-2-sulfanylidene-1,3-thiazolidin-5-ylidene]indol-1-yl]ethanoate | ethyl 2-[(3Z)-2-oxo-3-[4-oxo-3-(pyridine-3-carbonylamino)-2-sulfanylidene-1,3-thiazolidin-5-ylidene]indol-1-yl]acetate
Type:
Small organic molecule
Emp. Form.:
C21H16N4O5S2
Mol. Mass.:
468.506
SMILES:
CCOC(=O)CN1C(=O)\C(=C2/SC(=S)N(NC(=O)c3cccnc3)C2=O)c2ccccc12