Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
DNA (cytosine-5)-methyltransferase 3A
Ligand
BDBM46240
Substrate
n/a
Meas. Tech.
SAR Selectivity Analysis of small molecule inhibitors of PEST using pCAP in a fluorescence assay
IC50
7560±n/a nM
Citation
 PubChem, PC SAR Selectivity Analysis of small molecule inhibitors of PEST using pCAP in a fluorescence assay PubChem Bioassay (2010)[AID]
PubChem, PC SAR Selectivity Analysis of small molecule inhibitors of PEST using pCAP in a fluorescence assay PubChem Bioassay (2010)[AID] More Info.:
Target
Name:
DNA (cytosine-5)-methyltransferase 3A
Synonyms:
DNA (cytosine-5)-methyltransferase 3A | DNA MTase HsaIIIA | DNA methyltransferase HsaIIIA | DNM3A_HUMAN | DNMT3A | DNMT3A2/3L complex | M.HsaIIIA | tyrosine-protein phosphatase non-receptor type 12 isoform 2
Type:
PROTEIN
Mol. Mass.:
101857.24
Organism:
Homo sapiens (Human)
Description:
ChEMBL_1510405
Residue:
912
Sequence:
MPAMPSSGPGDTSSSAAEREEDRKDGEEQEEPRGKEERQEPSTTARKVGRPGRKRKHPPVESGDTPKDPAVISKSPSMAQDSGASELLPNGDLEKRSEPQPEEGSPAGGQKGGAPAEGEGAAETLPEASRAVENGCCTPKEGRGAPAEAGKEQKETNIESMKMEGSRGRLRGGLGWESSLRQRPMPRLTFQAGDPYYISKRKRDEWLARWKREAEKKAKVIAGMNAVEENQGPGESQKVEEASPPAVQQPTDPASPTVATTPEPVGSDAGDKNATKAGDDEPEYEDGRGFGIGELVWGKLRGFSWWPGRIVSWWMTGRSRAAEGTRWVMWFGDGKFSVVCVEKLMPLSSFCSAFHQATYNKQPMYRKAIYEVLQVASSRAGKLFPVCHDSDESDTAKAVEVQNKPMIEWALGGFQPSGPKGLEPPEEEKNPYKEVYTDMWVEPEAAAYAPPPPAKKPRKSTAEKPKVKEIIDERTRERLVYEVRQKCRNIEDICISCGSLNVTLEHPLFVGGMCQNCKNCFLECAYQYDDDGYQSYCTICCGGREVLMCGNNNCCRCFCVECVDLLVGPGAAQAAIKEDPWNCYMCGHKGTYGLLRRREDWPSRLQMFFANNHDQEFDPPKVYPPVPAEKRKPIRVLSLFDGIATGLLVLKDLGIQVDRYIASEVCEDSITVGMVRHQGKIMYVGDVRSVTQKHIQEWGPFDLVIGGSPCNDLSIVNPARKGLYEGTGRLFFEFYRLLHDARPKEGDDRPFFWLFENVVAMGVSDKRDISRFLESNPVMIDAKEVSAAHRARYFWGNLPGMNRPLASTVNDKLELQECLEHGRIAKFSKVRTITTRSNSIKQGKDQHFPVFMNEKEDILWCTEMERVFGFPVHYTDVSNMSRLARQRLLGRSWSVPVIRHLFAPLKEYFACV
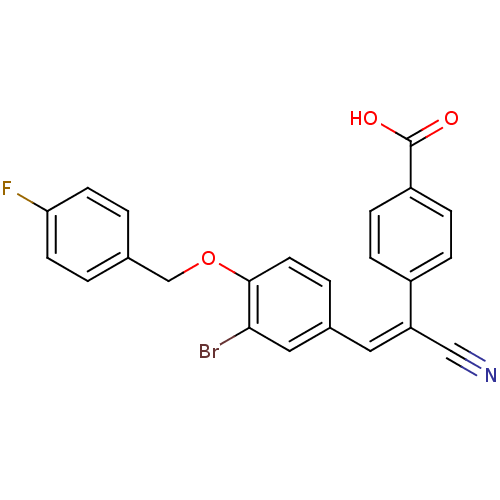
Inhibitor
Name:
BDBM46240
Synonyms:
4-[(E)-2-[3-bromanyl-4-[(4-fluorophenyl)methoxy]phenyl]-1-cyano-ethenyl]benzoic acid | 4-[(E)-2-[3-bromo-4-(4-fluorobenzyl)oxy-phenyl]-1-cyano-vinyl]benzoic acid | 4-[(E)-2-[3-bromo-4-[(4-fluorophenyl)methoxy]phenyl]-1-cyanoethenyl]benzoic acid | MLS-0412109.0001 | cid_2240797
Type:
Small organic molecule
Emp. Form.:
C23H15BrFNO3
Mol. Mass.:
452.273
SMILES:
OC(=O)c1ccc(cc1)C(=C/c1ccc(OCc2ccc(F)cc2)c(Br)c1)\C#N