Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Glycogen synthase kinase-3 alpha
Ligand
BDBM350085
Substrate
n/a
Meas. Tech.
ChEMBL_1852141 (CHEMBL4352765)
Ki
33±n/a nM
Citation
 Knegtel, R; Charrier, JD; Durrant, S; Davis, C; O'Donnell, M; Storck, P; MacCormick, S; Kay, D; Pinder, J; Virani, A; Twin, H; Griffiths, M; Reaper, P; Littlewood, P; Young, S; Golec, J; Pollard, J Rational Design of 5-(4-(Isopropylsulfonyl)phenyl)-3-(3-(4-((methylamino)methyl)phenyl)isoxazol-5-yl)pyrazin-2-amine (VX-970, M6620): Optimization of Intra- and Intermolecular Polar Interactions of a New Ataxia Telangiectasia Mutated and Rad3-Related (ATR) Kinase Inhibitor. J Med Chem 62:5547-5561 (2019) [PubMed] Article
Knegtel, R; Charrier, JD; Durrant, S; Davis, C; O'Donnell, M; Storck, P; MacCormick, S; Kay, D; Pinder, J; Virani, A; Twin, H; Griffiths, M; Reaper, P; Littlewood, P; Young, S; Golec, J; Pollard, J Rational Design of 5-(4-(Isopropylsulfonyl)phenyl)-3-(3-(4-((methylamino)methyl)phenyl)isoxazol-5-yl)pyrazin-2-amine (VX-970, M6620): Optimization of Intra- and Intermolecular Polar Interactions of a New Ataxia Telangiectasia Mutated and Rad3-Related (ATR) Kinase Inhibitor. J Med Chem 62:5547-5561 (2019) [PubMed] Article More Info.:
Target
Name:
Glycogen synthase kinase-3 alpha
Synonyms:
GSK-3 alpha | GSK3A | GSK3A_HUMAN | Glycogen synthase kinase 3 alpha (GSKalpha) | Glycogen synthase kinase-3 | Glycogen synthase kinase-3 alpha | Glycogen synthase kinase-3 alpha (GSK3 Alpha) | Glycogen synthase kinase-3 alpha (GSK3A) | Glycogen synthase kinase-3 alpha (GSK3alpha)
Type:
Enzyme
Mol. Mass.:
50991.79
Organism:
Homo sapiens (Human)
Description:
P49840
Residue:
483
Sequence:
MSGGGPSGGGPGGSGRARTSSFAEPGGGGGGGGGGPGGSASGPGGTGGGKASVGAMGGGVGASSSGGGPGGSGGGGSGGPGAGTSFPPPGVKLGRDSGKVTTVVATLGQGPERSQEVAYTDIKVIGNGSFGVVYQARLAETRELVAIKKVLQDKRFKNRELQIMRKLDHCNIVRLRYFFYSSGEKKDELYLNLVLEYVPETVYRVARHFTKAKLTIPILYVKVYMYQLFRSLAYIHSQGVCHRDIKPQNLLVDPDTAVLKLCDFGSAKQLVRGEPNVSYICSRYYRAPELIFGATDYTSSIDVWSAGCVLAELLLGQPIFPGDSGVDQLVEIIKVLGTPTREQIREMNPNYTEFKFPQIKAHPWTKVFKSRTPPEAIALCSSLLEYTPSSRLSPLEACAHSFFDELRCLGTQLPNNRPLPPLFNFSAGELSIQPSLNAILIPPHLRSPAGTTTLTPSSQALTETPTSSDWQSTDATPTLTNSS
Inhibitor
Name:
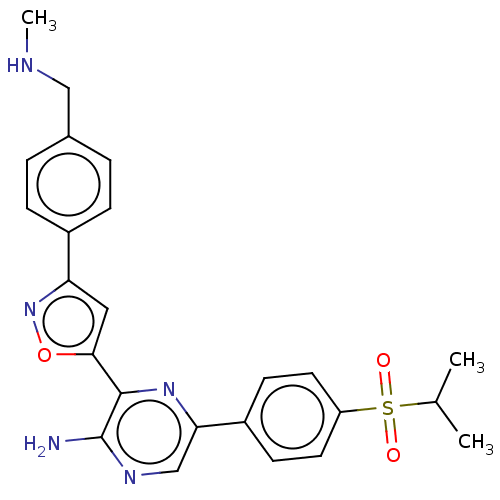
BDBM350085
Synonyms:
3-[3-[4-[dideuterio(methylamino)methyl]phenyl]isoxazol-5-yl]-5-(4-isopropylsulfonylphenyl)pyrazin-2-amine | BDBM50226746 | US10208027, Compound II-1 | US10208027, Compound II-2 | US10208027, Compound II-3 | US10208027, Compound II-4 | US10479784, Compound IIA-7 | US10822331, Cmpd II-4 | US10961232, Compound IIA-7 | US11787781, Compound A
Type:
Small organic molecule
Emp. Form.:
C24H25N5O3S
Mol. Mass.:
463.552
SMILES:
CNCc1ccc(cc1)-c1cc(on1)-c1nc(cnc1N)-c1ccc(cc1)S(=O)(=O)C(C)C