Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Cytochrome P450 3A4
Ligand
BDBM50517935
Substrate
n/a
Meas. Tech.
ChEMBL_1870101 (CHEMBL4371268)
EC50
89000±n/a nM
Citation
 Hanson, AM; Perera, KLIS; Kim, J; Pandey, RK; Sweeney, N; Lu, X; Imhoff, A; Mackinnon, AC; Wargolet, AJ; Van Hart, RM; Frick, KM; Donaldson, WA; Sem, DS A-C Estrogens as Potent and Selective Estrogen Receptor-Beta Agonists (SERBAs) to Enhance Memory Consolidation under Low-Estrogen Conditions. J Med Chem 61:4720-4738 (2018) [PubMed] Article
Hanson, AM; Perera, KLIS; Kim, J; Pandey, RK; Sweeney, N; Lu, X; Imhoff, A; Mackinnon, AC; Wargolet, AJ; Van Hart, RM; Frick, KM; Donaldson, WA; Sem, DS A-C Estrogens as Potent and Selective Estrogen Receptor-Beta Agonists (SERBAs) to Enhance Memory Consolidation under Low-Estrogen Conditions. J Med Chem 61:4720-4738 (2018) [PubMed] Article More Info.:
Target
Name:
Cytochrome P450 3A4
Synonyms:
Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase
Type:
Enzyme
Mol. Mass.:
57349.57
Organism:
Homo sapiens (Human)
Description:
n/a
Residue:
503
Sequence:
MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMFDMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISIAEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYSMDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICVFPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSIIFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVVNETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFSKKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLGGLLQPEKPVVLKVESRDGTVSGA
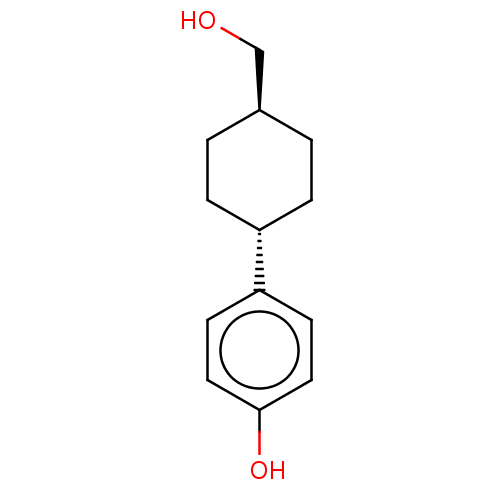
Inhibitor
Name:
BDBM50517935
Synonyms:
CHEMBL4526434
Type:
Small organic molecule
Emp. Form.:
C13H18O2
Mol. Mass.:
206.2808
SMILES:
OC[C@H]1CC[C@@H](CC1)c1ccc(O)cc1 |r,wU:5.8,wD:2.1,(30.01,-26.44,;29.24,-27.77,;27.7,-27.77,;26.93,-26.43,;25.39,-26.43,;24.62,-27.76,;25.39,-29.1,;26.93,-29.1,;23.08,-27.76,;22.32,-26.42,;20.78,-26.42,;20.01,-27.76,;18.47,-27.76,;20.79,-29.09,;22.32,-29.09,)|