Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Amyloid-beta precursor protein
Ligand
BDBM50323206
Substrate
n/a
Meas. Tech.
ChEMBL_1888363 (CHEMBL4390040)
IC50
>50000±n/a nM
Citation
 Sirimangkalakitti, N; Juliawaty, LD; Hakim, EH; Waliana, I; Saito, N; Koyama, K; Kinoshita, K Naturally occurring biflavonoids with amyloid ? aggregation inhibitory activity for development of anti-Alzheimer agents. Bioorg Med Chem Lett 29:1994-1997 (2019) [PubMed] Article
Sirimangkalakitti, N; Juliawaty, LD; Hakim, EH; Waliana, I; Saito, N; Koyama, K; Kinoshita, K Naturally occurring biflavonoids with amyloid ? aggregation inhibitory activity for development of anti-Alzheimer agents. Bioorg Med Chem Lett 29:1994-1997 (2019) [PubMed] Article More Info.:
Target
Name:
Amyloid-beta precursor protein
Synonyms:
A4 | A4_HUMAN | ABPP | AD1 | AICD-50 | AICD-57 | AICD-59 | AID(50) | AID(57) | AID(59) | APP | APPI | Alzheimer disease amyloid protein | Amyloid beta A4 protein | Amyloid beta Protein | Amyloid beta protein (sAPPbeta) | Amyloid beta protein Abeta(1-42) | Amyloid intracellular domain 50 | Amyloid intracellular domain 57 | Amyloid intracellular domain 59 | Amyloid protein (Abeta42b) | Amyloid β-protein (Aβ42) | Beta amyloid A4 protein | Beta-APP40 | Beta-APP42 | Beta-amyloid protein 40 | Beta-amyloid protein 42 | C31 | C83 | C99 | CVAP | Cerebral vascular amyloid peptide | Gamma Secretase | Gamma-CTF(50) | Gamma-CTF(57) | Gamma-CTF(59) | Gamma-secretase | Gamma-secretase C-terminal fragment 50 | Gamma-secretase C-terminal fragment 57 | Gamma-secretase C-terminal fragment 59 | P3(40) | P3(42) | PN-II | PreA4 | Protease nexin-II | S-APP-alpha | S-APP-beta | Soluble APP-alpha | Soluble APP-beta | beta-Amyloid Precursor Protein (APP)
Type:
Single-pass type I membrane protein
Mol. Mass.:
86890.41
Organism:
Homo sapiens (Human)
Description:
P05067
Residue:
770
Sequence:
MLPGLALLLLAAWTARALEVPTDGNAGLLAEPQIAMFCGRLNMHMNVQNGKWDSDPSGTKTCIDTKEGILQYCQEVYPELQITNVVEANQPVTIQNWCKRGRKQCKTHPHFVIPYRCLVGEFVSDALLVPDKCKFLHQERMDVCETHLHWHTVAKETCSEKSTNLHDYGMLLPCGIDKFRGVEFVCCPLAEESDNVDSADAEEDDSDVWWGGADTDYADGSEDKVVEVAEEEEVAEVEEEEADDDEDDEDGDEVEEEAEEPYEEATERTTSIATTTTTTTESVEEVVREVCSEQAETGPCRAMISRWYFDVTEGKCAPFFYGGCGGNRNNFDTEEYCMAVCGSAMSQSLLKTTQEPLARDPVKLPTTAASTPDAVDKYLETPGDENEHAHFQKAKERLEAKHRERMSQVMREWEEAERQAKNLPKADKKAVIQHFQEKVESLEQEAANERQQLVETHMARVEAMLNDRRRLALENYITALQAVPPRPRHVFNMLKKYVRAEQKDRQHTLKHFEHVRMVDPKKAAQIRSQVMTHLRVIYERMNQSLSLLYNVPAVAEEIQDEVDELLQKEQNYSDDVLANMISEPRISYGNDALMPSLTETKTTVELLPVNGEFSLDDLQPWHSFGADSVPANTENEVEPVDARPAADRGLTTRPGSGLTNIKTEEISEVKMDAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVVIATVIVITLVMLKKKQYTSIHHGVVEVDAAVTPEERHLSKMQQNGYENPTYKFFEQMQN
Inhibitor
Name:
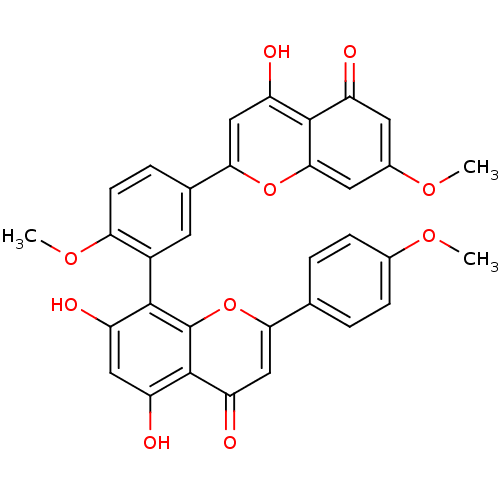
BDBM50323206
Synonyms:
CHEMBL208908 | sciadopitisin | sciadopitysin
Type:
Small organic molecule
Emp. Form.:
C33H24O10
Mol. Mass.:
580.5377
SMILES:
COc1ccc(cc1)-c1cc(=O)c2c(O)cc(O)c(-c3cc(ccc3OC)-c3cc(O)c4c(cc(OC)cc4=O)o3)c2o1 |(10.61,-33.01,;9.27,-32.25,;7.94,-33.02,;7.94,-34.56,;6.61,-35.34,;5.28,-34.57,;5.27,-33.03,;6.6,-32.26,;3.95,-35.34,;3.95,-36.87,;2.63,-37.63,;2.63,-39.17,;1.31,-36.87,;-.03,-37.66,;-.03,-39.2,;-1.36,-36.89,;-1.36,-35.35,;-2.69,-34.58,;-.02,-34.58,;-.02,-33.04,;-1.36,-32.27,;-1.35,-30.73,;-.03,-29.96,;1.31,-30.73,;1.31,-32.27,;2.65,-33.04,;3.98,-32.27,;-2.68,-29.97,;-2.68,-28.42,;-4.03,-27.64,;-4.03,-26.1,;-5.36,-28.42,;-5.36,-29.97,;-6.7,-30.74,;-8.03,-29.97,;-9.37,-30.74,;-10.7,-29.97,;-8.03,-28.42,;-6.7,-27.64,;-6.7,-26.1,;-4.02,-30.74,;1.3,-35.35,;2.62,-34.57,)|