Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Inositol 1,4,5-trisphosphate receptor type 3
Ligand
BDBM50078572
Substrate
n/a
Meas. Tech.
ChEBML_90087
pH
7.4±n/a
Kd
1.2±n/a nM
Comments
extracted
Citation
More Info.:
Target
Name:
Inositol 1,4,5-trisphosphate receptor type 3
Synonyms:
ITPR3 | ITPR3_HUMAN | Inositol 1,4,5-trisphosphate receptor | Inositol 1,4,5-trisphosphate receptor type 3
Type:
PROTEIN
Mol. Mass.:
304100.07
Organism:
Homo sapiens (Human)
Description:
ChEMBL_90252
Residue:
2671
Sequence:
MSEMSSFLHIGDIVSLYAEGSVNGFISTLGLVDDRCVVEPAAGDLDNPPKKFRDCLFKVCPMNRYSAQKQYWKAKQTKQDKEKIADVVLLQKLQHAAQMEQKQNDTENKKVHGDVVKYGSVIQLLHMKSNKYLTVNKRLPALLEKNAMRVTLDATGNEGSWLFIQPFWKLRSNGDNVVVGDKVILNPVNAGQPLHASNYELSDNAGCKEVNSVNCNTSWKINLFMQFRDHLEEVLKGGDVVRLFHAEQEKFLTCDEYKGKLQVFLRTTLRQSATSATSSNALWEVEVVHHDPCRGGAGHWNGLYRFKHLATGNYLAAEENPSYKGDASDPKAAGMGAQGRTGRRNAGEKIKYCLVAVPHGNDIASLFELDPTTLQKTDSFVPRNSYVRLRHLCTNTWIQSTNVPIDIEEERPIRLMLGTCPTKEDKEAFAIVSVPVSEIRDLDFANDASSMLASAVEKLNEGFISQNDRRFVIQLLEDLVFFVSDVPNNGQNVLDIMVTKPNRERQKLMREQNILKQVFGILKAPFREKGGEGPLVRLEELSDQKNAPYQHMFRLCYRVLRHSQEDYRKNQEHIAKQFGMMQSQIGYDILAEDTITALLHNNRKLLEKHITKTEVETFVSLVRKNREPRFLDYLSDLCVSNHIAIPVTQELICKCVLDPKNSDILIRTELRPVKEMAQSHEYLSIEYSEEEVWLTWTDKNNEHHEKSVRQLAQEARAGNAHDENVLSYYRYQLKLFARMCLDRQYLAIDEISQQLGVDLIFLCMADEMLPFDLRASFCHLMLHVHVDRDPQELVTPVKFARLWTEIPTAITIKDYDSNLNASRDDKKNKFANTMEFVEDYLNNVVSEAVPFANEEKNKLTFEVVSLAHNLIYFGFYSFSELLRLTRTLLGIIDCVQGPPAMLQAYEDPGGKNVRRSIQGVGHMMSTMVLSRKQSVFSAPSLSAGASAAEPLDRSKFEENEDIVVMETKLKILEILQFILNVRLDYRISYLLSVFKKEFVEVFPMQDSGADGTAPAFDSTTANMNLDRIGEQAEAMFGVGKTSSMLEVDDEGGRMFLRVLIHLTMHDYAPLVSGALQLLFKHFSQRQEAMHTFKQVQLLISAQDVENYKVIKSELDRLRTMVEKSELWVDKKGSGKGEEVEAGAAKDKKERPTDEEGFLHPPGEKSSENYQIVKGILERLNKMCGVGEQMRKKQQRLLKNMDAHKVMLDLLQIPYDKGDAKMMEILRYTHQFLQKFCAGNPGNQALLHKHLHLFLTPGLLEAETMQHIFLNNYQLCSEISEPVLQHFVHLLATHGRHVQYLDFLHTVIKAEGKYVKKCQDMIMTELTNAGDDVVVFYNDKASLAHLLDMMKAARDGVEDHSPLMYHISLVDLLAACAEGKNVYTEIKCTSLLPLEDVVSVVTHEDCITEVKMAYVNFVNHCYVDTEVEMKEIYTSNHIWTLFENFTLDMARVCSKREKRVADPTLEKYVLSVVLDTINAFFSSPFSENSTSLQTHQTIVVQLLQSTTRLLECPWLQQQHKGSVEACIRTLAMVAKGRAILLPMDLDAHISSMLSSGASCAAAAQRNASSYKATTRAFPRVTPTANQWDYKNIIEKLQDIITALEERLKPLVQAELSVLVDVLHWPELLFLEGSEAYQRCESGGFLSKLIQHTKDLMESEEKLCIKVLRTLQQMLLKKTKYGDRGNQLRKMLLQNYLQNRKSTSRGDLPDPIGTGLDPDWSAIAATQCRLDKEGATKLVCDLITSTKNEKIFQESIGLAIHLLDGGNTEIQKSFHNLMMSDKKSERFFKVLHDRMKRAQQETKSTVAVNMNDLGSQPHEDREPVDPTTKGRVASFSIPGSSSRYSLGPSLRRGHEVSERVQSSEMGTSVLIMQPILRFLQLLCENHNRDLQNFLRCQNNKTNYNLVCETLQFLDIMCGSTTGGLGLLGLYINEDNVGLVIQTLETLTEYCQGPCHENQTCIVTHESNGIDIITALILNDISPLCKYRMDLVLQLKDNASKLLLALMESRHDSENAERILISLRPQELVDVIKKAYLQEEERENSEVSPREVGHNIYILALQLSRHNKQLQHLLKPVKRIQEEEAEGISSMLSLNNKQLSQMLKSSAPAQEEEEDPLAYYENHTSQIEIVRQDRSMEQIVFPVPGICQFLTEETKHRLFTTTEQDEQGSKVSDFFDQSSFLHNEMEWQRKLRSMPLIYWFSRRMTLWGSISFNLAVFINIIIAFFYPYMEGASTGVLDSPLISLLFWILICFSIAALFTKRYSIRPLIVALILRSIYYLGIGPTLNILGALNLTNKIVFVVSFVGNRGTFIRGYKAMVMDMEFLYHVGYILTSVLGLFAHELFYSILLFDLIYREETLFNVIKSVTRNGRSILLTALLALILVYLFSIVGFLFLKDDFILEVDRLPNNHSTASPLGMPHGAAAFVDTCSGDKMDCVSGLSVPEVLEEDRELDSTERACDTLLMCIVTVMNHGLRNGGGVGDILRKPSKDESLFPARVVYDLLFFFIVIIIVLNLIFGVIIDTFADLRSEKQKKEEILKTTCFICGLERDKFDNKTVSFEEHIKLEHNMWNYLYFIVLVRVKNKTDYTGPESYVAQMIKNKNLDWFPRMRAMSLVSNEGEGEQNEIRILQDKLNSTMKLVSHLTAQLNELKEQMTEQRKRRQRLGFVDVQNCISR
Inhibitor
Name:
BDBM50078572
Synonyms:
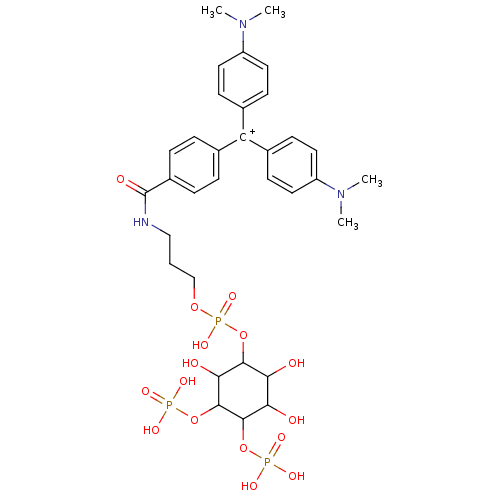
CHEMBL172534 | CHEMBL297496 | {4-[(4-Dimethylamino-phenyl)-(4-{3-[hydroxy-((1R,2R,3S,4R,5R,6S)-2,3,6-trihydroxy-4,5-bis-phosphonooxy-cyclohexyloxy)-phosphoryloxy]-propylcarbamoyl}-phenyl)-methylene]-cyclohexa-2,5-dienylidene}-dimethyl-ammonium; chloride
Type:
Small organic molecule
Emp. Form.:
C33H45N3O16P3
Mol. Mass.:
832.6416
SMILES:
CN(C)c1ccc(cc1)[C+](c1ccc(cc1)N(C)C)c1ccc(cc1)C(=O)NCCCOP(O)(=O)OC1C(O)C(O)C(OP(O)(O)=O)C(OP(O)(O)=O)C1O