Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Cytochrome P450 3A4
Ligand
BDBM50538670
Substrate
n/a
Meas. Tech.
ChEMBL_1973673 (CHEMBL4606491)
EC50
50000±n/a nM
Citation
 Janetka, JW; Hopper, AT; Yang, Z; Barks, J; Dhason, MS; Wang, Q; Sibley, LD Optimizing Pyrazolopyrimidine Inhibitors of Calcium Dependent Protein Kinase 1 for Treatment of Acute and Chronic Toxoplasmosis. J Med Chem 63:6144-6163 (2020) [PubMed] Article
Janetka, JW; Hopper, AT; Yang, Z; Barks, J; Dhason, MS; Wang, Q; Sibley, LD Optimizing Pyrazolopyrimidine Inhibitors of Calcium Dependent Protein Kinase 1 for Treatment of Acute and Chronic Toxoplasmosis. J Med Chem 63:6144-6163 (2020) [PubMed] Article More Info.:
Target
Name:
Cytochrome P450 3A4
Synonyms:
Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase
Type:
Enzyme
Mol. Mass.:
57349.57
Organism:
Homo sapiens (Human)
Description:
n/a
Residue:
503
Sequence:
MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMFDMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISIAEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYSMDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICVFPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSIIFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVVNETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFSKKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLGGLLQPEKPVVLKVESRDGTVSGA
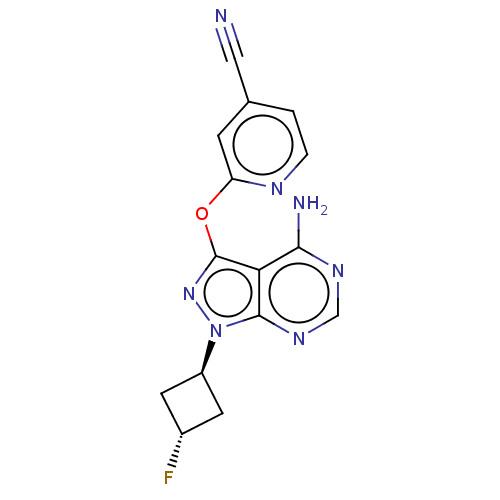
Inhibitor
Name:
BDBM50538670
Synonyms:
CHEMBL4642579
Type:
Small organic molecule
Emp. Form.:
C15H12FN7O
Mol. Mass.:
325.3005
SMILES:
Nc1ncnc2n(nc(Oc3cc(ccn3)C#N)c12)[C@H]1C[C@H](F)C1 |r,wU:19.21,wD:21.24,(4.07,-26.23,;4.07,-27.77,;2.74,-28.54,;2.74,-30.08,;4.08,-30.85,;5.42,-30.07,;6.89,-30.54,;7.79,-29.29,;6.87,-28.04,;7.34,-26.58,;8.84,-26.25,;9.3,-24.78,;10.8,-24.45,;11.85,-25.59,;11.38,-27.06,;9.87,-27.39,;11.25,-22.99,;11.72,-21.52,;5.41,-28.53,;7.38,-32,;8.75,-32.69,;8.06,-34.07,;8.55,-35.53,;6.68,-33.38,)|