Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Protein phosphatase 1B
Ligand
BDBM50097633
Substrate
n/a
Meas. Tech.
ChEBML_161755
IC50
94000±n/a nM
Citation
 Frydrychowski, VA; Urbanek, RA; Dounay, AB; Forsyth, CJ Importance of the C28-C38 hydrophobic domain of okadaic acid for potent inhibition of protein serine-threonine phosphatases 1 and 2A. Bioorg Med Chem Lett 11:647-9 (2001) [PubMed] Article
Frydrychowski, VA; Urbanek, RA; Dounay, AB; Forsyth, CJ Importance of the C28-C38 hydrophobic domain of okadaic acid for potent inhibition of protein serine-threonine phosphatases 1 and 2A. Bioorg Med Chem Lett 11:647-9 (2001) [PubMed] Article More Info.:
Target
Name:
Protein phosphatase 1B
Synonyms:
PP2C-beta | PP2CB | PPM1B | PPM1B_HUMAN | Protein phosphatase 1B | Protein phosphatase 2C beta | Protein phosphatase 2C isoform beta
Type:
PROTEIN
Mol. Mass.:
52620.37
Organism:
Homo sapiens (Human)
Description:
ChEMBL_161756
Residue:
479
Sequence:
MGAFLDKPKTEKHNAHGAGNGLRYGLSSMQGWRVEMEDAHTAVVGIPHGLEDWSFFAVYDGHAGSRVANYCSTHLLEHITTNEDFRAAGKSGSALELSVENVKNGIRTGFLKIDEYMRNFSDLRNGMDRSGSTAVGVMISPKHIYFINCGDSRAVLYRNGQVCFSTQDHKPCNPREKERIQNAGGSVMIQRVNGSLAVSRALGDYDYKCVDGKGPTEQLVSPEPEVYEILRAEEDEFIILACDGIWDVMSNEELCEYVKSRLEVSDDLENVCNWVVDTCLHKGSRDNMSIVLVCFSNAPKVSDEAVKKDSELDKHLESRVEEIMEKSGEEGMPDLAHVMRILSAENIPNLPPGGGLAGKRNVIEAVYSRLNPHRESDGASDEAEESGSQGKLVEALRQMRINHRGNYRQLLEEMLTSYRLAKVEGEESPAEPAATATSSNSDAGNPVTMQESHTESESGLAELDSSNEDAGTKMSGEKI
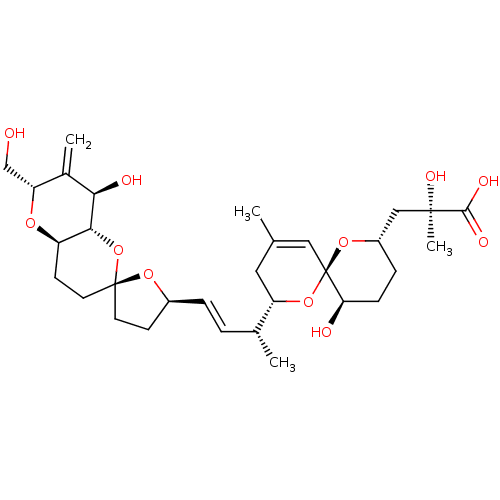
Inhibitor
Name:
BDBM50097633
Synonyms:
CHEMBL2368224 | okadaic acid analogue
Type:
Small organic molecule
Emp. Form.:
C31H46O11
Mol. Mass.:
594.6903
SMILES:
[H][C@@]1(CC[C@@]2(CC[C@@]3([H])O[C@]([H])(CO)C(=C)[C@@H](O)[C@]3([H])O2)O1)\C=C\[C@@H](C)[C@]1([H])CC(C)=C[C@@]2(O[C@]([H])(C[C@@](C)(O)C(O)=O)CC[C@H]2O)O1 |c:33|