Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
DNA gyrase subunit A/B
Ligand
BDBM50554221
Substrate
n/a
Meas. Tech.
ChEMBL_2048803 (CHEMBL4703502)
IC50
2.7±n/a nM
Citation
 Ushiyama, F; Amada, H; Mihara, Y; Takeuchi, T; Tanaka-Yamamoto, N; Mima, M; Kamitani, M; Wada, R; Tamura, Y; Endo, M; Masuko, A; Takata, I; Hitaka, K; Sugiyama, H; Ohtake, N Lead optimization of 8-(methylamino)-2-oxo-1,2-dihydroquinolines as bacterial type II topoisomerase inhibitors. Bioorg Med Chem 28:0 (2020) [PubMed] Article
Ushiyama, F; Amada, H; Mihara, Y; Takeuchi, T; Tanaka-Yamamoto, N; Mima, M; Kamitani, M; Wada, R; Tamura, Y; Endo, M; Masuko, A; Takata, I; Hitaka, K; Sugiyama, H; Ohtake, N Lead optimization of 8-(methylamino)-2-oxo-1,2-dihydroquinolines as bacterial type II topoisomerase inhibitors. Bioorg Med Chem 28:0 (2020) [PubMed] Article More Info.:
Target
Name:
DNA gyrase subunit A/B
Synonyms:
DNA Gyrase | DNA gyrase A/B
Type:
A2B2 tetramer
Mol. Mass.:
n/a
Description:
n/a
Components:
This complex has 2 components.
Component 1
Name:
DNA gyrase subunit A
Synonyms:
DNA gyrase | DNA gyrase subunit A (gyrA) | GYRA_ECOLI | gyrA | hisW | nalA | parD
Type:
Enzyme Subunit
Mol. Mass.:
96935.15
Organism:
Escherichia coli (strain K12)
Description:
n/a
Residue:
875
Sequence:
MSDLAREITPVNIEEELKSSYLDYAMSVIVGRALPDVRDGLKPVHRRVLYAMNVLGNDWNKAYKKSARVVGDVIGKYHPHGDSAVYDTIVRMAQPFSLRYMLVDGQGNFGSIDGDSAAAMRYTEIRLAKIAHELMADLEKETVDFVDNYDGTEKIPDVMPTKIPNLLVNGSSGIAVGMATNIPPHNLTEVINGCLAYIDDEDISIEGLMEHIPGPDFPTAAIINGRRGIEEAYRTGRGKVYIRARAEVEVDAKTGRETIIVHEIPYQVNKARLIEKIAELVKEKRVEGISALRDESDKDGMRIVIEVKRDAVGEVVLNNLYSQTQLQVSFGINMVALHHGQPKIMNLKDIIAAFVRHRREVVTRRTIFELRKARDRAHILEALAVALANIDPIIELIRHAPTPAEAKTALVANPWQLGNVAAMLERAGDDAARPEWLEPEFGVRDGLYYLTEQQAQAILDLRLQKLTGLEHEKLLDEYKELLDQIAELLRILGSADRLMEVIREELELVREQFGDKRRTEITANSADINLEDLITQEDVVVTLSHQGYVKYQPLSEYEAQRRGGKGKSAARIKEEDFIDRLLVANTHDHILCFSSRGRVYSMKVYQLPEATRGARGRPIVNLLPLEQDERITAILPVTEFEEGVKVFMATANGTVKKTVLTEFNRLRTAGKVAIKLVDGDELIGVDLTSGEDEVMLFSAEGKVVRFKESSVRAMGCNTTGVRGIRLGEGDKVVSLIVPRGDGAILTATQNGYGKRTAVAEYPTKSRATKGVISIKVTERNGLVVGAVQVDDCDQIMMITDAGTLVRTRVSEISIVGRNTQGVILIRTAEDENVVGLQRVAEPVDEEDLDTIDGSAAEGDDEIAPEVDVDDEPEEE
Component 2
Name:
DNA gyrase subunit B
Synonyms:
DNA gyrase subunit B | DNA gyrase subunit B (gyrB) | GYRB_ECOLI | Type IIA topoisomerase subunit GyrB | acrB | cou | gyrB | himB | hisU | nalC | parA | pcbA
Type:
Enzyme Subunit
Mol. Mass.:
89941.28
Organism:
Escherichia coli (strain K12)
Description:
P0AES6
Residue:
804
Sequence:
MSNSYDSSSIKVLKGLDAVRKRPGMYIGDTDDGTGLHHMVFEVVDNAIDEALAGHCKEIIVTIHADNSVSVQDDGRGIPTGIHPEEGVSAAEVIMTVLHAGGKFDDNSYKVSGGLHGVGVSVVNALSQKLELVIQREGKIHRQIYEHGVPQAPLAVTGETEKTGTMVRFWPSLETFTNVTEFEYEILAKRLRELSFLNSGVSIRLRDKRDGKEDHFHYEGGIKAFVEYLNKNKTPIHPNIFYFSTEKDGIGVEVALQWNDGFQENIYCFTNNIPQRDGGTHLAGFRAAMTRTLNAYMDKEGYSKKAKVSATGDDAREGLIAVVSVKVPDPKFSSQTKDKLVSSEVKSAVEQQMNELLAEYLLENPTDAKIVVGKIIDAARAREAARRAREMTRRKGALDLAGLPGKLADCQERDPALSELYLVEGDSAGGSAKQGRNRKNQAILPLKGKILNVEKARFDKMLSSQEVATLITALGCGIGRDEYNPDKLRYHSIIIMTDADVDGSHIRTLLLTFFYRQMPEIVERGHVYIAQPPLYKVKKGKQEQYIKDDEAMDQYQISIALDGATLHTNASAPALAGEALEKLVSEYNATQKMINRMERRYPKAMLKELIYQPTLTEADLSDEQTVTRWVNALVSELNDKEQHGSQWKFDVHTNAEQNLFEPIVRVRTHGVDTDYPLDHEFITGGEYRRICTLGEKLRGLLEEDAFIERGERRQPVASFEQALDWLVKESRRGLSIQRYKGLGEMNPEQLWETTMDPESRRMLRVTVKDAIAADQLFTTLMGDAVEPRRAFIEENALKAANIDI
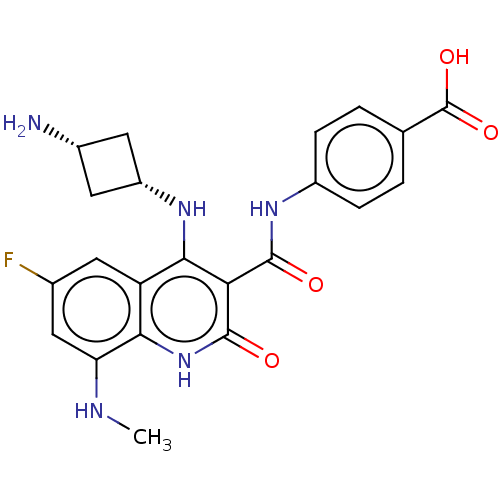
Inhibitor
Name:
BDBM50554221
Synonyms:
CHEMBL4744595
Type:
Small organic molecule
Emp. Form.:
C23H24FN5O6
Mol. Mass.:
485.465
SMILES:
OC=O.CNc1cc(F)cc2c(N[C@@H]3C[C@H](N)C3)c(C(=O)Nc3ccc(cc3)C(O)=O)c(=O)[nH]c12 |r,wU:13.11,15.14,(34.6,-13.1,;33.26,-13.88,;33.26,-15.42,;26.62,-4.17,;27.96,-4.94,;27.96,-6.48,;26.64,-7.25,;26.63,-8.79,;25.3,-9.56,;27.97,-9.56,;29.31,-8.8,;30.65,-9.56,;30.65,-11.1,;29.32,-11.88,;27.84,-11.48,;27.44,-12.96,;26.11,-13.74,;28.93,-13.36,;31.99,-8.79,;33.33,-9.55,;33.33,-11.09,;34.66,-8.78,;36,-9.54,;35.99,-11.09,;37.33,-11.85,;38.66,-11.08,;38.65,-9.53,;37.32,-8.77,;40,-11.84,;41.33,-11.07,;40,-13.38,;31.99,-7.23,;33.32,-6.46,;30.64,-6.45,;29.3,-7.24,)|