Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
DNA topoisomerase 4 subunit A/B
Ligand
BDBM50577579
Substrate
n/a
Meas. Tech.
ChEMBL_2130603 (CHEMBL4840032)
IC50
380±n/a nM
Citation
 Lu, Y; Vibhute, S; Li, L; Okumu, A; Ratigan, SC; Nolan, S; Papa, JL; Mann, CA; English, A; Chen, A; Seffernick, JT; Koci, B; Duncan, LR; Roth, B; Cummings, JE; Slayden, RA; Lindert, S; McElroy, CA; Wozniak, DJ; Yalowich, J; Mitton-Fry, MJ Optimization of TopoIV Potency, ADMET Properties, and hERG Inhibition of 5-Amino-1,3-dioxane-Linked Novel Bacterial Topoisomerase Inhibitors: Identification of a Lead with J Med Chem 64:15214-15249 (2021) [PubMed] Article
Lu, Y; Vibhute, S; Li, L; Okumu, A; Ratigan, SC; Nolan, S; Papa, JL; Mann, CA; English, A; Chen, A; Seffernick, JT; Koci, B; Duncan, LR; Roth, B; Cummings, JE; Slayden, RA; Lindert, S; McElroy, CA; Wozniak, DJ; Yalowich, J; Mitton-Fry, MJ Optimization of TopoIV Potency, ADMET Properties, and hERG Inhibition of 5-Amino-1,3-dioxane-Linked Novel Bacterial Topoisomerase Inhibitors: Identification of a Lead with J Med Chem 64:15214-15249 (2021) [PubMed] Article More Info.:
Target
Name:
DNA topoisomerase 4 subunit A/B
Synonyms:
Topoisomerase IV
Type:
n/a
Mol. Mass.:
n/a
Description:
ASSAY_ID of ChEMBL is 2012758
Components:
This complex has 2 components.
Component 1
Name:
DNA topoisomerase 4 subunit A
Synonyms:
PARC_STAAU | Topoisomerase IV subunit A | grlA | parC
Type:
PROTEIN
Mol. Mass.:
91040.14
Organism:
Staphylococcus aureus
Description:
ChEMBL_340188
Residue:
800
Sequence:
MSEIIQDLSLEDVLGDRFGRYSKYIIQERALPDVRDGLKPVQRRILYAMYSSGNTHDKNFRKSAKTVGDVIGQYHPHGDFSVYKAMVRLSQDWKLRHVLIEMHGNNGSIDNDPPAAMRYTEAKLSLLAEELLRDINKETVSFIPNYDDTTLEPMVLPSRFPNLLVNGSTGISAGYATDIPPHNLAEVIQATLKYIDNPDITVNQLMKYIKGPDFPTGGIIQGIDGIKKAYESGKGRIIVRSKVEEETLRNGRKQLIITEIPYEVNKSSLVKRIDELRADKKVDGIVEVRDETDRTGLRIAIELKKDVNSESIKNYLYKNSDLQISYNFNMVAISDGRPKLMGIRQIIDSYLNHQIEVVANRTKFELDNAEKRMHIVEGLIKALSILDKVIELIRSSKNKRDAKENLIEVFEFTEEQAEAIVMLQLYRLTNTDIVALEGEHKELEALIKQLRHILDNHDALLNVIKEELNEIKKKFKSERLSLIEAEIEEIKIDKEVMVPSEEVILSMTRHGYIKRTSIRSFNASGVEDIGLKDGDSLLKHQEVNTQDTVLVFTNKGRYLFIPVHKLADIRWKELGQHVSQIVPIEEDEVVINVFNEKDFNTDAFYVFATQNGMIKKSTVPLFKTTRFNKPLIATKVKENDDLISVMRFEKDQLITVITNKGMSLTYNTSELSDTGLRAAGVKSINLKAEDFVVMTEGVSENDTILMATQRGSLKRISFKILQVAKRAQRGITLLKELKKNPHRIVAAHVVTGEHSQYTLYSKSNEEHGLINDIHKSEQYTNGSFIVDTDDFGEVIDMYIS
Component 2
Name:
DNA topoisomerase 4 subunit B
Synonyms:
DNA topoisomerase 4 subunit B | DNA topoisomerase 4 subunit B (parE) | PARE_STAA8 | Topoisomerase IV subunit B | grlB | parE
Type:
Enzyme
Mol. Mass.:
74365.92
Organism:
Staphylococcus aureus
Description:
Q2FYS5
Residue:
663
Sequence:
MNKQNNYSDDSIQVLEGLEAVRKRPGMYIGSTDKRGLHHLVYEIVDNSVDEVLNGYGNEIDVTINKDGSISIEDNGRGMPTGIHKSGKPTVEVIFTVLHAGGKFGQGGYKTSGGLHGVGASVVNALSEWLEVEIHRDGNIYHQSFKNGGSPSSGLVKKGKTKKTGTKVTFKPDDTIFKASTSFNFDVLSERLQESAFLLKNLKITLNDLRSGKERQEHYHYEEGIKEFVSYVNEGKEVLHDVATFSGEANGIEVDVAFQYNDQYSESILSFVNNVRTKDGGTHEVGFKTAMTRVFNDYARRINELKTKDKNLDGNDIREGLTAVVSVRIPEELLQFEGQTKSKLGTSEARSAVDSVVADKLPFYLEEKGQLSKSLVKKAIKAQQAREAARKAREDARSGKKNKRKDTLLSGKLTPAQSKNTEKNELYLVEGDSAGGSAKLGRDRKFQAILPLRGKVINTEKARLEDIFKNEEINTIIHTIGAGVGTDFKIEDSNYNRVIIMTDADTDGAHIQVLLLTFFFKYMKPLVQAGRVFIALPPLYKLEKGKGKTKRVEYAWTDEELNKLQKELGKGFTLQRYKGLGEMNPEQLWETTMNPETRTLIRVQVEDEVRSSKRVTTLMGDKVQPRREWIEKHVEFGMQEDQSILDNSEVQVLENDQFDEEEI
Inhibitor
Name:
BDBM50577579
Synonyms:
CHEMBL4867964
Type:
Small organic molecule
Emp. Form.:
C24H26FN3O6
Mol. Mass.:
471.4781
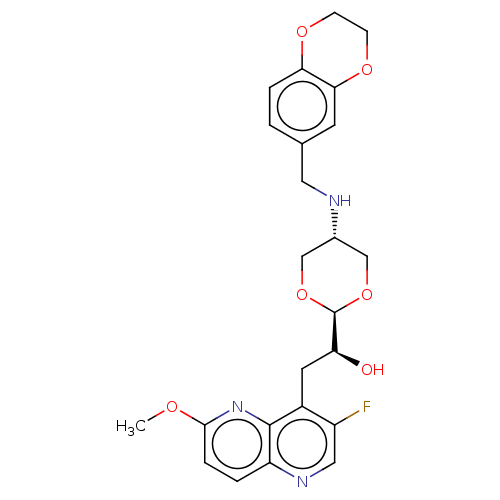
SMILES:
[H][C@@]1(OC[C@@H](CO1)NCc1ccc2OCCOc2c1)[C@@H](O)Cc1c(F)cnc2ccc(OC)nc12 |r,wU:19.22,wD:4.7,1.0,(12.1,-14.99,;10.78,-14.23,;9.44,-13.47,;9.44,-11.93,;10.77,-11.15,;12.11,-11.93,;12.11,-13.46,;10.77,-9.61,;12.1,-8.84,;13.43,-9.61,;13.43,-11.15,;14.76,-11.92,;16.09,-11.14,;17.42,-11.9,;18.76,-11.13,;18.76,-9.59,;17.42,-8.82,;16.09,-9.6,;14.76,-8.84,;10.78,-15.77,;12.12,-16.53,;9.46,-16.54,;9.46,-18.08,;10.8,-18.84,;12.13,-18.07,;10.8,-20.39,;9.47,-21.17,;8.14,-20.4,;6.81,-21.18,;5.47,-20.41,;5.47,-18.86,;4.14,-18.09,;4.14,-16.55,;6.8,-18.09,;8.14,-18.86,)|