Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Neuronal acetylcholine receptor; alpha2/beta4
Ligand
BDBM50581248
Substrate
n/a
Meas. Tech.
ChEMBL_2150172 (CHEMBL5034634)
IC50
>10000±n/a nM
Citation
 Zheng, N; Christensen, SB; Dowell, C; Purushottam, L; Skalicky, JJ; McIntosh, JM; Chou, DH Discovery of Methylene Thioacetal-Incorporated ?-RgIA Analogues as Potent and Stable Antagonists of the Human ?9?10 Nicotinic Acetylcholine Receptor for the Treatment of Neuropathic Pain. J Med Chem 64:9513-9524 (2021) [PubMed] Article
Zheng, N; Christensen, SB; Dowell, C; Purushottam, L; Skalicky, JJ; McIntosh, JM; Chou, DH Discovery of Methylene Thioacetal-Incorporated ?-RgIA Analogues as Potent and Stable Antagonists of the Human ?9?10 Nicotinic Acetylcholine Receptor for the Treatment of Neuropathic Pain. J Med Chem 64:9513-9524 (2021) [PubMed] Article More Info.:
Target
Name:
Neuronal acetylcholine receptor; alpha2/beta4
Synonyms:
Neuronal acetylcholine receptor protein alpha-2 subunit/subunit beta-4 | Neuronal acetylcholine receptor subunit alpha-2/beta-4
Type:
n/a
Mol. Mass.:
n/a
Description:
ASSAY_ID of ChEMBL is 2150172
Components:
This complex has 2 components.
Component 1
Name:
Neuronal acetylcholine receptor subunit beta-4
Synonyms:
ACHB4_HUMAN | CHRNB4 | Cholinergic, Nicotinic Alpha6Beta3Beta4Alpha5 | Integrin alpha-5/Neuronal acetylcholine receptor subunit alpha-3/Neuronal acetylcholine receptor subunit beta-2/Neuronal acetylcholine receptor subunit beta-4 | Nicotinic acetylcholine receptor alpha6/alpha3/beta4
Type:
Enzyme Catalytic Domain
Mol. Mass.:
56388.51
Organism:
Homo sapiens (Human)
Description:
Cholinergic, Nicotinic Alpha6Beta3Beta4Alpha5 0 HUMAN::P30926
Residue:
498
Sequence:
MRRAPSLVLFFLVALCGRGNCRVANAEEKLMDDLLNKTRYNNLIRPATSSSQLISIKLQLSLAQLISVNEREQIMTTNVWLKQEWTDYRLTWNSSRYEGVNILRIPAKRIWLPDIVLYNNADGTYEVSVYTNLIVRSNGSVLWLPPAIYKSACKIEVKYFPFDQQNCTLKFRSWTYDHTEIDMVLMTPTASMDDFTPSGEWDIVALPGRRTVNPQDPSYVDVTYDFIIKRKPLFYTINLIIPCVLTTLLAILVFYLPSDCGEKMTLCISVLLALTFFLLLISKIVPPTSLDVPLIGKYLMFTMVLVTFSIVTSVCVLNVHHRSPSTHTMAPWVKRCFLHKLPTFLFMKRPGPDSSPARAFPPSKSCVTKPEATATSTSPSNFYGNSMYFVNPASAASKSPAGSTPVAIPRDFWLRSSGRFRQDVQEALEGVSFIAQHMKNDDEDQSVVEDWKYVAMVVDRLFLWVFMFVCVLGTVGLFLPPLFQTHAASEGPYAAQRD
Component 2
Name:
Neuronal acetylcholine receptor subunit alpha-2
Synonyms:
ACHA2_HUMAN | CHRNA2 | Neuronal acetylcholine receptor; alpha2/beta2
Type:
Enzyme Catalytic Domain
Mol. Mass.:
59759.69
Organism:
Homo sapiens (Human)
Description:
Cholinergic, Nicotinic Alpha2Beta2 0 HUMAN::Q15822
Residue:
529
Sequence:
MGPSCPVFLSFTKLSLWWLLLTPAGGEEAKRPPPRAPGDPLSSPSPTALPQGGSHTETEDRLFKHLFRGYNRWARPVPNTSDVVIVRFGLSIAQLIDVDEKNQMMTTNVWLKQEWSDYKLRWNPTDFGNITSLRVPSEMIWIPDIVLYNNADGEFAVTHMTKAHLFSTGTVHWVPPAIYKSSCSIDVTFFPFDQQNCKMKFGSWTYDKAKIDLEQMEQTVDLKDYWESGEWAIVNATGTYNSKKYDCCAEIYPDVTYAFVIRRLPLFYTINLIIPCLLISCLTVLVFYLPSDCGEKITLCISVLLSLTVFLLLITEIIPSTSLVIPLIGEYLLFTMIFVTLSIVITVFVLNVHHRSPSTHTMPHWVRGALLGCVPRWLLMNRPPPPVELCHPLRLKLSPSYHWLESNVDAEEREVVVEEEDRWACAGHVAPSVGTLCSHGHLHSGASGPKAEALLQEGELLLSPHMQKALEGVHYIADHLRSEDADSSVKEDWKYVAMVIDRIFLWLFIIVCFLGTIGLFLPPFLAGMI
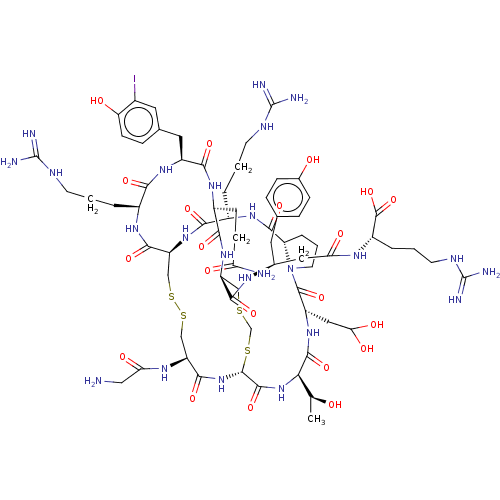
Inhibitor
Name:
BDBM50581248
Synonyms:
CHEMBL5090753
Type:
Small organic molecule
Emp. Form.:
C69H105IN24O21S4
Mol. Mass.:
1861.885
SMILES:
C[C@H](O)[C@H]1NC(=O)[C@@H]2NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]3CCCN3C(=O)[C@H](CC(O)O)NC1=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)c(I)c1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CSCS2)C(=O)N[C@H](CC(=O)N[C@@H](CCCNC(N)=N)C(O)=O)Cc1ccc(O)cc1)NC(=O)CN |r|