Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Mitofusin-2
Ligand
BDBM50581381
Substrate
n/a
Meas. Tech.
ChEMBL_2150923 (CHEMBL5035385)
EC50
3.8±n/a nM
Citation
 Dang, X; Williams, SB; Devanathan, S; Franco, A; Fu, L; Bernstein, PR; Walters, D; Dorn, GW Pharmacophore-Based Design of Phenyl-[hydroxycyclohexyl] Cycloalkyl-Carboxamide Mitofusin Activators with Improved Neuronal Activity. J Med Chem 64:12506-12524 (2021) [PubMed] Article
Dang, X; Williams, SB; Devanathan, S; Franco, A; Fu, L; Bernstein, PR; Walters, D; Dorn, GW Pharmacophore-Based Design of Phenyl-[hydroxycyclohexyl] Cycloalkyl-Carboxamide Mitofusin Activators with Improved Neuronal Activity. J Med Chem 64:12506-12524 (2021) [PubMed] Article More Info.:
Target
Name:
Mitofusin-2
Synonyms:
3.6.5.- | CPRP1 | KIAA0214 | MFN2 | MFN2_HUMAN | Synonyms=CPRP1 | Transmembrane GTPase MFN2
Type:
PROTEIN
Mol. Mass.:
86406.66
Organism:
Homo sapiens
Description:
ChEMBL_119900
Residue:
757
Sequence:
MSLLFSRCNSIVTVKKNKRHMAEVNASPLKHFVTAKKKINGIFEQLGAYIQESATFLEDTYRNAELDPVTTEEQVLDVKGYLSKVRGISEVLARRHMKVAFFGRTSNGKSTVINAMLWDKVLPSGIGHTTNCFLRVEGTDGHEAFLLTEGSEEKRSAKTVNQLAHALHQDKQLHAGSLVSVMWPNSKCPLLKDDLVLMDSPGIDVTTELDSWIDKFCLDADVFVLVANSESTLMQTEKHFFHKVSERLSRPNIFILNNRWDASASEPEYMEEVRRQHMERCTSFLVDELGVVDRSQAGDRIFFVSAKEVLNARIQKAQGMPEGGGALAEGFQVRMFEFQNFERRFEECISQSAVKTKFEQHTVRAKQIAEAVRLIMDSLHMAAREQQVYCEEMREERQDRLKFIDKQLELLAQDYKLRIKQITEEVERQVSTAMAEEIRRLSVLVDDYQMDFHPSPVVLKVYKNELHRHIEEGLGRNMSDRCSTAITNSLQTMQQDMIDGLKPLLPVSVRSQIDMLVPRQCFSLNYDLNCDKLCADFQEDIEFHFSLGWTMLVNRFLGPKNSRRALMGYNDQVQRPIPLTPANPSMPPLPQGSLTQEEFMVSMVTGLASLTSRTSMGILVVGGVVWKAVGWRLIALSFGLYGLLYVYERLTWTTKAKERAFKRQFVEHASEKLQLVISYTGSNCSHQVQQELSGTFAHLCQQVDVTRENLEQEIAAMNKKIEVLDSLQSKAKLLRNKAGWLDSELNMFTHQYLQPSR
Inhibitor
Name:
BDBM50581381
Synonyms:
CHEMBL5088708
Type:
Small organic molecule
Emp. Form.:
C19H27NO2
Mol. Mass.:
301.4232
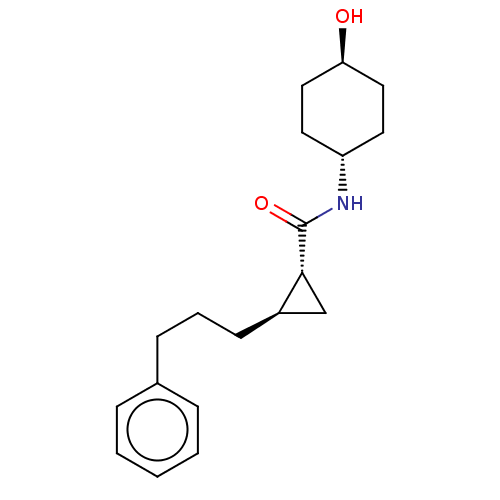
SMILES:
O[C@H]1CC[C@@H](CC1)NC(=O)[C@@H]1C[C@H]1CCCc1ccccc1 |r,wU:10.10,4.7,wD:1.0,12.14,(24.24,-3.49,;25.57,-4.26,;25.57,-5.8,;26.9,-6.58,;28.23,-5.81,;28.24,-4.27,;26.91,-3.5,;29.56,-6.58,;30.9,-5.81,;30.9,-4.27,;32.23,-6.58,;33,-7.91,;33.77,-6.57,;35.1,-5.8,;36.43,-6.57,;37.77,-5.79,;39.1,-6.56,;39.1,-8.1,;40.43,-8.87,;41.76,-8.09,;41.76,-6.54,;40.42,-5.78,)|