Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Beta-lactamase TEM
Ligand
BDBM50149466
Substrate
n/a
Meas. Tech.
ChEMBL_41201 (CHEMBL876650)
IC50
1±0 nM
Citation
 Tabei, K; Feng, X; Venkatesan, AM; Abe, T; Hideki, U; Mansour, TS; Siegel, MM Mechanism of inactivation of beta-lactamases by novel 6-methylidene penems elucidated using electrospray ionization mass spectrometry. J Med Chem 47:3674-88 (2004) [PubMed] Article
Tabei, K; Feng, X; Venkatesan, AM; Abe, T; Hideki, U; Mansour, TS; Siegel, MM Mechanism of inactivation of beta-lactamases by novel 6-methylidene penems elucidated using electrospray ionization mass spectrometry. J Med Chem 47:3674-88 (2004) [PubMed] Article More Info.:
Target
Name:
Beta-lactamase TEM
Synonyms:
BLAT_ECOLX | Bacterial beta-lactamase TEM | Beta-lactamase (TEM-1) | Beta-lactamase TEM | Beta-lactamase TEM-1 | Beta-lactamase TEM-1b | Beta-lactamase TEM1D | Beta-lactamase TEM1E | Beta-lactamase TEM1F | TEM beta lactamase | TEM extended-spectrum beta-lactamase | TEM-1 beta-lactamase | bla
Type:
Enzyme
Mol. Mass.:
31512.33
Organism:
Escherichia coli
Description:
P62593
Residue:
286
Sequence:
MSIQHFRVALIPFFAAFCLPVFAHPETLVKVKDAEDQLGARVGYIELDLNSGKILESFRPEERFPMMSTFKVLLCGAVLSRVDAGQEQLGRRIHYSQNDLVEYSPVTEKHLTDGMTVRELCSAAITMSDNTAANLLLTTIGGPKELTAFLHNMGDHVTRLDRWEPELNEAIPNDERDTTMPAAMATTLRKLLTGELLTLASRQQLIDWMEADKVAGPLLRSALPAGWFIADKSGAGERGSRGIIAALGPDGKPSRIVVIYTTGSQATMDERNRQIAEIGASLIKHW
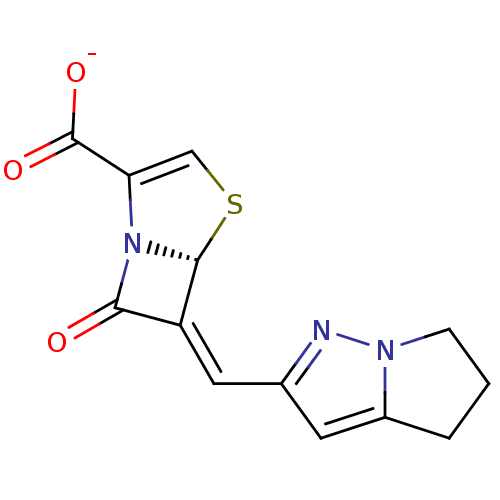
Inhibitor
Name:
BDBM50149466
Synonyms:
CHEMBL124416 | Sodium; (R)-6-[1-(5,6-dihydro-4H-pyrrolo[1,2-b]pyrazol-2-yl)-meth-(Z)-ylidene]-7-oxo-4-thia-1-aza-bicyclo[3.2.0]hept-2-ene-2-carboxylate
Type:
Small organic molecule
Emp. Form.:
C13H10N3O3S
Mol. Mass.:
288.302
SMILES:
[O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cc2CCCn2n1 |t:3|