Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Angiotensin-converting enzyme 2
Ligand
BDBM50582779
Substrate
n/a
Meas. Tech.
ChEMBL_2155625 (CHEMBL5040285)
IC50
18±n/a nM
Citation
 Sadremomtaz, A; Al-Dahmani, ZM; Ruiz-Moreno, AJ; Monti, A; Wang, C; Azad, T; Bell, JC; Doti, N; Velasco-Velázquez, MA; de Jong, D; de Jonge, J; Smit, J; Dömling, A; van Goor, H; Groves, MR Synthetic Peptides That Antagonize the Angiotensin-Converting Enzyme-2 (ACE-2) Interaction with SARS-CoV-2 Receptor Binding Spike Protein. J Med Chem 65:2836-2847 (2022) [PubMed] Article
Sadremomtaz, A; Al-Dahmani, ZM; Ruiz-Moreno, AJ; Monti, A; Wang, C; Azad, T; Bell, JC; Doti, N; Velasco-Velázquez, MA; de Jong, D; de Jonge, J; Smit, J; Dömling, A; van Goor, H; Groves, MR Synthetic Peptides That Antagonize the Angiotensin-Converting Enzyme-2 (ACE-2) Interaction with SARS-CoV-2 Receptor Binding Spike Protein. J Med Chem 65:2836-2847 (2022) [PubMed] Article More Info.:
Target
Name:
Angiotensin-converting enzyme 2
Synonyms:
ACE-related carboxypeptidase | ACE2 | ACE2_HUMAN | ACEH | Angiotensin-converting enzyme homolog | Angiotensin-converting enzyme-related carboxypeptidase | Metalloprotease MPROT15
Type:
Enzyme
Mol. Mass.:
92448.86
Organism:
Homo sapiens (Human)
Description:
n/a
Residue:
805
Sequence:
MSSSSWLLLSLVAVTAAQSTIEEQAKTFLDKFNHEAEDLFYQSSLASWNYNTNITEENVQNMNNAGDKWSAFLKEQSTLAQMYPLQEIQNLTVKLQLQALQQNGSSVLSEDKSKRLNTILNTMSTIYSTGKVCNPDNPQECLLLEPGLNEIMANSLDYNERLWAWESWRSEVGKQLRPLYEEYVVLKNEMARANHYEDYGDYWRGDYEVNGVDGYDYSRGQLIEDVEHTFEEIKPLYEHLHAYVRAKLMNAYPSYISPIGCLPAHLLGDMWGRFWTNLYSLTVPFGQKPNIDVTDAMVDQAWDAQRIFKEAEKFFVSVGLPNMTQGFWENSMLTDPGNVQKAVCHPTAWDLGKGDFRILMCTKVTMDDFLTAHHEMGHIQYDMAYAAQPFLLRNGANEGFHEAVGEIMSLSAATPKHLKSIGLLSPDFQEDNETEINFLLKQALTIVGTLPFTYMLEKWRWMVFKGEIPKDQWMKKWWEMKREIVGVVEPVPHDETYCDPASLFHVSNDYSFIRYYTRTLYQFQFQEALCQAAKHEGPLHKCDISNSTEAGQKLFNMLRLGKSEPWTLALENVVGAKNMNVRPLLNYFEPLFTWLKDQNKNSFVGWSTDWSPYADQSIKVRISLKSALGDKAYEWNDNEMYLFRSSVAYAMRQYFLKVKNQMILFGEEDVRVANLKPRISFNFFVTAPKNVSDIIPRTEVEKAIRMSRSRINDAFRLNDNSLEFLGIQPTLGPPNQPPVSIWLIVFGVVMGVIVVGIVILIFTGIRDRKKKNKARSGENPYASIDISKGENNPGFQNTDDVQTSF
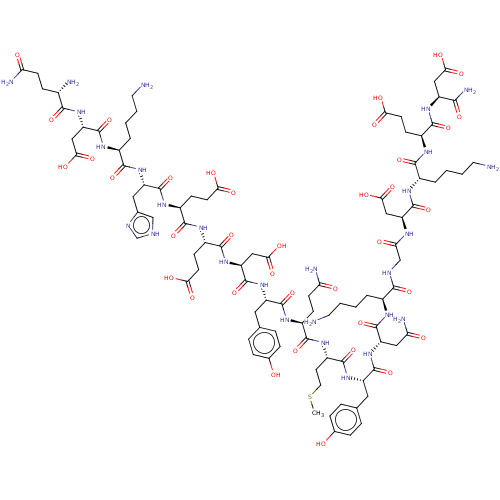
Inhibitor
Name:
BDBM50582779
Synonyms:
CHEMBL5081573
Type:
Small organic molecule
Emp. Form.:
C94H139N27O37S
Mol. Mass.:
2271.333
SMILES:
CSCC[C@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CCC(N)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(N)=O |r|