Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Cathepsin D
Ligand
BDBM50173732
Substrate
n/a
Meas. Tech.
ChEMBL_321034 (CHEMBL883666)
pH
4.5±n/a
Ki
>6000±n/a nM
Comments
extracted
Citation
 Ersmark, K; Nervall, M; Hamelink, E; Janka, LK; Clemente, JC; Dunn, BM; Blackman, MJ; Samuelsson, B; Aqvist, J; Hallberg, A Synthesis of malarial plasmepsin inhibitors and prediction of binding modes by molecular dynamics simulations. J Med Chem 48:6090-106 (2005) [PubMed] Article
Ersmark, K; Nervall, M; Hamelink, E; Janka, LK; Clemente, JC; Dunn, BM; Blackman, MJ; Samuelsson, B; Aqvist, J; Hallberg, A Synthesis of malarial plasmepsin inhibitors and prediction of binding modes by molecular dynamics simulations. J Med Chem 48:6090-106 (2005) [PubMed] Article More Info.:
Target
Name:
Cathepsin D
Synonyms:
CATD_HUMAN | CPSD | CTSD | Cathepsin D [Precursor] | Cathepsin D heavy chain | Cathepsin D light chain | Cathepsin D precursor
Type:
Enzyme
Mol. Mass.:
44551.72
Organism:
Homo sapiens (Human)
Description:
Human proCathepsin D (SwissProt accession number P07339) was expressed in Sf9 cells, purified, and autoactivated.
Residue:
412
Sequence:
MQPSSLLPLALCLLAAPASALVRIPLHKFTSIRRTMSEVGGSVEDLIAKGPVSKYSQAVPAVTEGPIPEVLKNYMDAQYYGEIGIGTPPQCFTVVFDTGSSNLWVPSIHCKLLDIACWIHHKYNSDKSSTYVKNGTSFDIHYGSGSLSGYLSQDTVSVPCQSASSASALGGVKVERQVFGEATKQPGITFIAAKFDGILGMAYPRISVNNVLPVFDNLMQQKLVDQNIFSFYLSRDPDAQPGGELMLGGTDSKYYKGSLSYLNVTRKAYWQVHLDQVEVASGLTLCKEGCEAIVDTGTSLMVGPVDEVRELQKAIGAVPLIQGEYMIPCEKVSTLPAITLKLGGKGYKLSPEDYTLKVSQAGKTLCLSGFMGMDIPPPSGPLWILGDVFIGRYYTVFDRDNNRVGFAEAARL
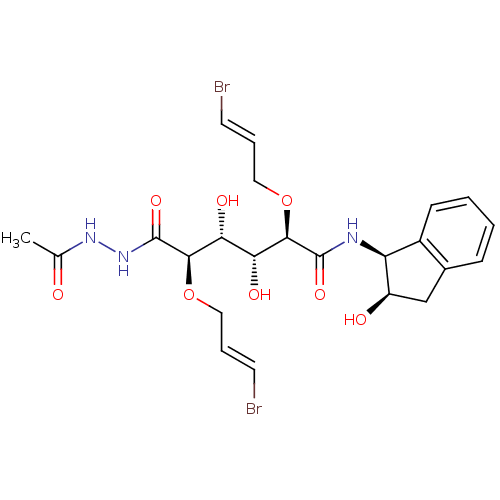
Inhibitor
Name:
BDBM50173732
Synonyms:
(2R,3R,4R,5R)-6-(N'-Acetyl-hydrazino)-2,5-bis-((E)-3-bromo-allyloxy)-3,4-dihydroxy-6-oxo-hexanoic acid ((1S,2R)-2-hydroxy-indan-1-yl)-amide | CHEMBL382274
Type:
Small organic molecule
Emp. Form.:
C23H29Br2N3O8
Mol. Mass.:
635.3
SMILES:
CC(=O)NNC(=O)[C@H](OC\C=C\Br)[C@H](O)[C@@H](O)[C@@H](OC\C=C\Br)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12