Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Glycogen phosphorylase, muscle form
Ligand
BDBM50175880
Substrate
n/a
Meas. Tech.
ChEMBL_334360 (CHEMBL859105)
IC50
580000±n/a nM
Citation
More Info.:
Target
Name:
Glycogen phosphorylase, muscle form
Synonyms:
Glycogen Phosphorylase (PYGM) | Glycogen phosphorylase a (RMGPa) | Glycogen phosphorylase, muscle form | Myophosphorylase | PYGM | PYGM_RABIT
Type:
Enzyme
Mol. Mass.:
97296.32
Organism:
Oryctolagus cuniculus (rabbit)
Description:
Phosphorylation of Ser-15 converts phosphorylase B (unphosphorylated) to phosphorylase A.
Residue:
843
Sequence:
MSRPLSDQEKRKQISVRGLAGVENVTELKKNFNRHLHFTLVKDRNVATPRDYYFALAHTVRDHLVGRWIRTQQHYYEKDPKRIYYLSLEFYMGRTLQNTMVNLALENACDEATYQLGLDMEELEEIEEDAGLGNGGLGRLAACFLDSMATLGLAAYGYGIRYEFGIFNQKICGGWQMEEADDWLRYGNPWEKARPEFTLPVHFYGRVEHTSQGAKWVDTQVVLAMPYDTPVPGYRNNVVNTMRLWSAKAPNDFNLKDFNVGGYIQAVLDRNLAENISRVLYPNDNFFEGKELRLKQEYFVVAATLQDIIRRFKSSKFGCRDPVRTNFDAFPDKVAIQLNDTHPSLAIPELMRVLVDLERLDWDKAWEVTVKTCAYTNHTVLPEALERWPVHLLETLLPRHLQIIYEINQRFLNRVAAAFPGDVDRLRRMSLVEEGAVKRINMAHLCIAGSHAVNGVARIHSEILKKTIFKDFYELEPHKFQNKTNGITPRRWLVLCNPGLAEIIAERIGEEYISDLDQLRKLLSYVDDEAFIRDVAKVKQENKLKFAAYLEREYKVHINPNSLFDVQVKRIHEYKRQLLNCLHVITLYNRIKKEPNKFVVPRTVMIGGKAAPGYHMAKMIIKLITAIGDVVNHDPVVGDRLRVIFLENYRVSLAEKVIPAADLSEQISTAGTEASGTGNMKFMLNGALTIGTMDGANVEMAEEAGEENFFIFGMRVEDVDRLDQRGYNAQEYYDRIPELRQIIEQLSSGFFSPKQPDLFKDIVNMLMHHDRFKVFADYEEYVKCQERVSALYKNPREWTRMVIRNIATSGKFSSDRTIAQYAREIWGVEPSRQRLPAPDEKIP
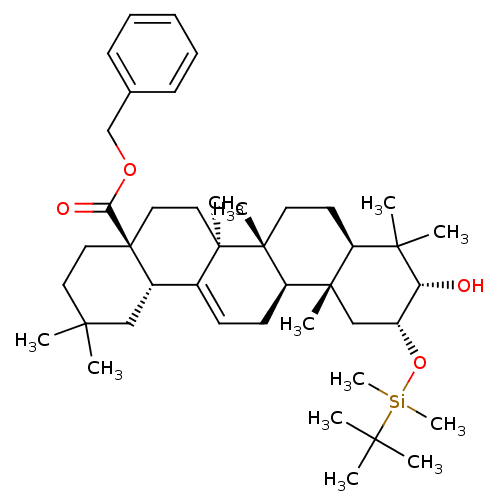
Inhibitor
Name:
BDBM50175880
Synonyms:
CHEMBL380974 | benzyl (4aS,6aS,6bR,8aR,10S,11R,12aR,12bR,14bS)-11-[(tert-butyldimethylsilyl)oxy]-10-hydroxy-2,2,6a,6b,9,9,12a-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-icosahydropicene-4a-carboxylate
Type:
Small organic molecule
Emp. Form.:
C43H68O4Si
Mol. Mass.:
677.0831
SMILES:
CC(C)(C)[Si](C)(C)O[C@@H]1C[C@@]2(C)[C@@H](CC[C@]3(C)[C@@H]2CC=C2[C@@H]4CC(C)(C)CC[C@@]4(CC[C@@]32C)C(=O)OCc2ccccc2)C(C)(C)[C@@H]1O |t:20|